Table of Contents
Definition of the Quality Audit
Quality Audit is defined as a systematic and independent examination to determine whether activities and related results comply with the planned arrangements and whether these arrangements are implemented effectively and are suitable to achieve objectives.
Quality audits are typically performed at defined intervals. Any failure in their proper implementation may be published publicly and may lead to a revocation of quality certification.
Types of Audit
- A first-party audit is an audit performed by an organization on itself i.e. an internal audit.
- A second party audit is an audit performed by one organization on its own behalf on another usually on a supplier by a customer.
- A third-party audit is an audit by an independent organization other than the customer on a supplier
Phases of Audit
Phase 1-Preparation: This phase precedes the actual review meeting. It is the responsibility of the chairman and presenter to organize the quality review and notify all those invited
Phase 2- The review meeting: The central phase of the quality review process is the review meeting itself. During the review meeting, the emphasis should be on error detection, in line with the criteria, and only limited discussion of corrective action should occur.
Phase 3- The Follow-Up Following the quality review meeting there should be a follow-up period during which the errors identified at the review that was committed to the follow-up action list are rectified and signed
Objectives of Quality Audit
Pharmaceutical manufacturers commonly audit as an effective mechanism to verify compliance with GMP regulations. The general objectives of a quality audit are as follows: To determine conformity or non-conformity of the quality system elements with specified requirements. To determine the effectiveness of the implemented system in meeting specified quality objectives. To afford an opportunity to improve the quality system. To provide Managers with information.
Principles of Auditing
Ethical Conduct: The foundation of Professionalism, Trust, Integrity, Confidentiality, and discretion are essential to auditing.
Fair Presentation: The obligation to report truthfully and accurately.
Due professional care: The application of diligence and judgment in auditing Independence: The basis for the impartiality of the audit and objectivity of the audit conclusions.
Evidence-based approach: The rational method for reaching reliable and reproducible audit.
Types of Quality Audit
- Adequacy audit/ document review
- Compliance audit/ on-site audit
- External audit
- Internal audit
- Product or process audit
Adequacy audit/ Document review
This is also known as a system or management audit and is normally documented system represented by the quality manual and the associated procedures adequately meets the need of the applicable standard.
Compliance audit/ On-site audit
This is the audit that seeks to establish the extent to which the documented system is implemented and observed by the workforce, i.e. are the people complying with the system
External Audit
This is an audit that a company performs on its own suppliers or subcontractors. The purpose of an external audit is to gain confidence in the partnership arrangement. This ensures that requirements are understood. There is a reduction of in-house Q.C testing of starting materials and reduces the risk of failure.
Internal Audit
This is the most important type of audit, which requires the company to look into its own systems, procedures, and activities in order to ascertain whether they are adequate and being compiled. It provides the management with information on whether or not their policies are being met if the system is as efficient and as effective as it should be and whether any changes are needed. It can provide a line of communication throughout the company and be a great motivator.
Product/ Process Audit
Product review refers to an in-depth examination of a particular product/service to evaluate whether it conforms to product specifications, performance standards, and customer requirements. Process audit refers to an analysis of elements of a process and appraisal of completeness, correctness of conditions, and probable effectiveness.
The Audit Life Cycle
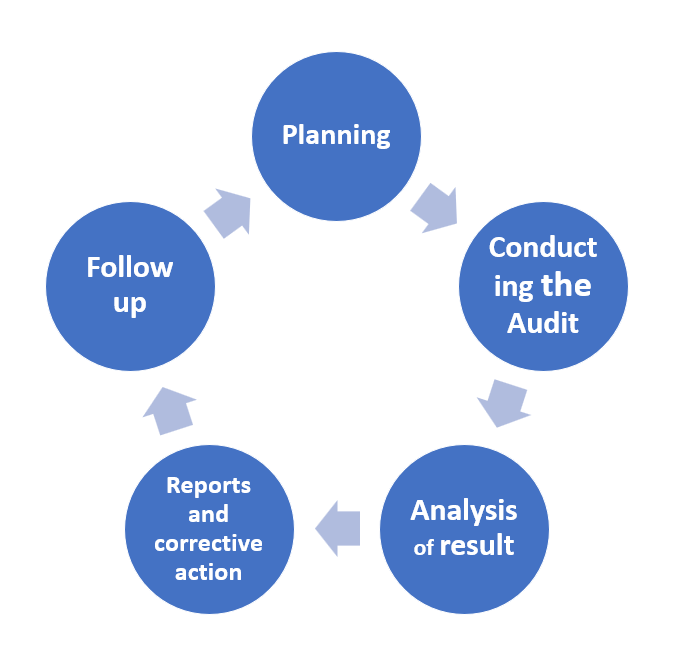
Audit Methods and Techniques
Audit Methods & Techniques are categorized based on the purpose of the audit.
Horizontal Auditing
It involves the examination of each functional area of an organization to verify adequacy and implementation of Quality. Used for internal system auditing and second & third party assessment when it is necessary to establish if a basic QMS has been installed and is being implemented and maintained. Each functional area is checked for conformance with quality system requirements, applicable to that area.
Vertical Auditing
It involves examining functional areas of an organization that are actively contributing to a specific work package or contractual requirement.
Random auditing
It examines the various aspects of an organization’s operation as determined by the auditor and the need to closely examine a particular actively or generally probe the system in a random manner.
Role of GMP Audit In Q.A And Q.C Programmes
- Auditor’s review of SOP’s employees’ practices and behavior
- Compare master specifications against compendia & regulatory requirements.
- Verify the test data and validation testing
- Validation test reports are compared against raw data.
- Verify corrective actions taken in reaction to an audit finding
GMP Regulation Format
The basic elements are derived from the following subparts of regulations:
- Organization and personnel
- Building and facilities Equipment
- Production and Processing controls
- Holding and distribution
- Lab controls
- Records and reports
- Returned and salvaged drug
Written Criteria & SOP have to be established defining which audit data or elements are to be considered in the assessment of program performance. Formal written SOPs should fully describe the details for carrying out the various audit functions like The responsibility for audit data review Personnel responsible for recommendation Decisions concerning corrective actions Effective use of written criteria to ensure that conditions and practices remain under a suitable state of control. SOPs should be established.
Planned periodic frequency
Each firm must establish the optimum time interval between audits based on several important factors like: -intended purpose -objectives, scope and depth -prior history of audit finding Two types of Visits can be done depending on the type of audit: Announced Visit Up-announced Visit
Personnel: The following personnel factors deserve systemic attention.
- Defining audit or Qualification: Auditors are selected based on their knowledge Their work experience in manufacturing and QC as well as years of first-hand dealing with GMP matters. Essential Auditor skill includes awareness of the firm’s SOPs and knowledge of its various departments.
- Documentation training skills & Experience: There are usually 2 formats
- Scientific principles, training in chemistry, engineering, statistics, and pharmaceutics.
- GMP training may include cumulative knowledge from reasonable years of experience. This knowledge comes from; daily activities and formal training sessions
- Audit teams: The personnel in the audit team are selected based on their experience and knowledge. The team is required to cover many different systems and a large amount of data. The composition of the team will vary depending upon the nature and scope of the audit. The leader is usually a senior auditor who has extensive knowledge of the firm’s operations & has strong leadership qualities. Tem size depends upon Firm size Total number of products manufactured & control systems
Reporting Audit Finding
Audit reports should contain complete details of the program detected. Corrective action is taken to eliminate problems and to measure the overall adequacy of the audit program There are two important reporting phases
- Preliminary reports during the audit
- Final report to the management
Benefits derived from Audit
The major benefits that are derived from Audits are as follows:
- Assuring GMP compliance
- Detecting potential problems
- Effecting programmed improvement
- Increasing management awareness
Audit Check
- Documentation work.
- QA/QC Issues.
- SOP Manuals.
- Building and Facilities.
- Fallure Investigation.
- Process Validation Program Master Records.
- Production and In-Process controls.
- Packaging and labeling of API’s and Intermediates.
- Equipment Processing.
- Storage and Distribution.
- Material Management.
- Housekeeping facilities
Make sure you also check our other amazing Article on: Standard Operating Procedure
