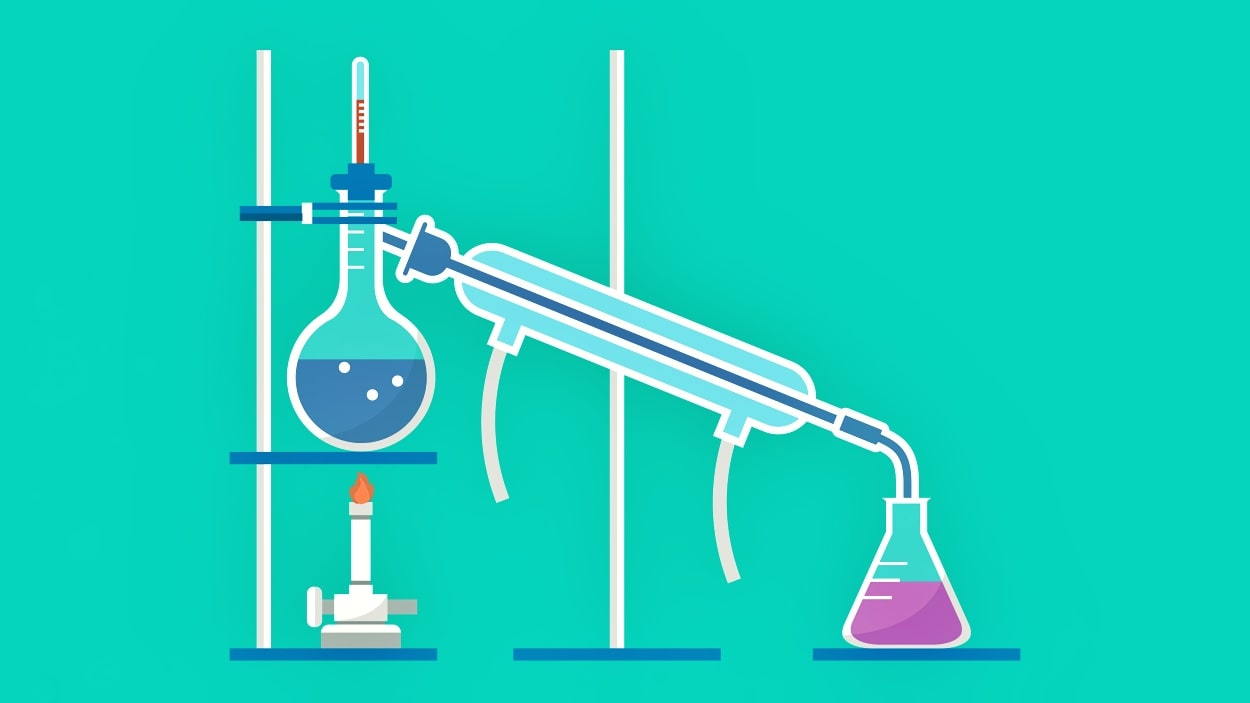
In this process, the vapor is removed from the system as soon as it is formed and condensed. Simple distillation is preferred for the purification and separation of liquids having high volatility. The Rayleigh Equation is useful in the analysis of simple distillation.
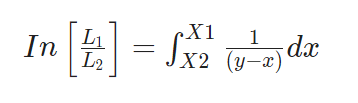
Where,
L1 and L2 are the total numbers of moles of the liquid still before and after distillation.
X1 and x2 are the mole fraction of more volatile components in L1 and L2 respectively
x and y are obtained by equilibrium diagram. The plot of x vs 1/y-x is drawn to get an integrated value by estimating the area under the curve between limits x1 and x2
Apparatus for Simple Distillation
It consists of a distillation flask or still in which the liquid which is to be distilled is boiled. A condenser where vapors are condensed into a liquid. Water is circulated through the jacket of the condenser. The condenser is attached to the receiver by an adapter. The receiver is used to collect liquid. A thermometer is also inserted into the cork and attached to the flask. The whole unit is made of glass.
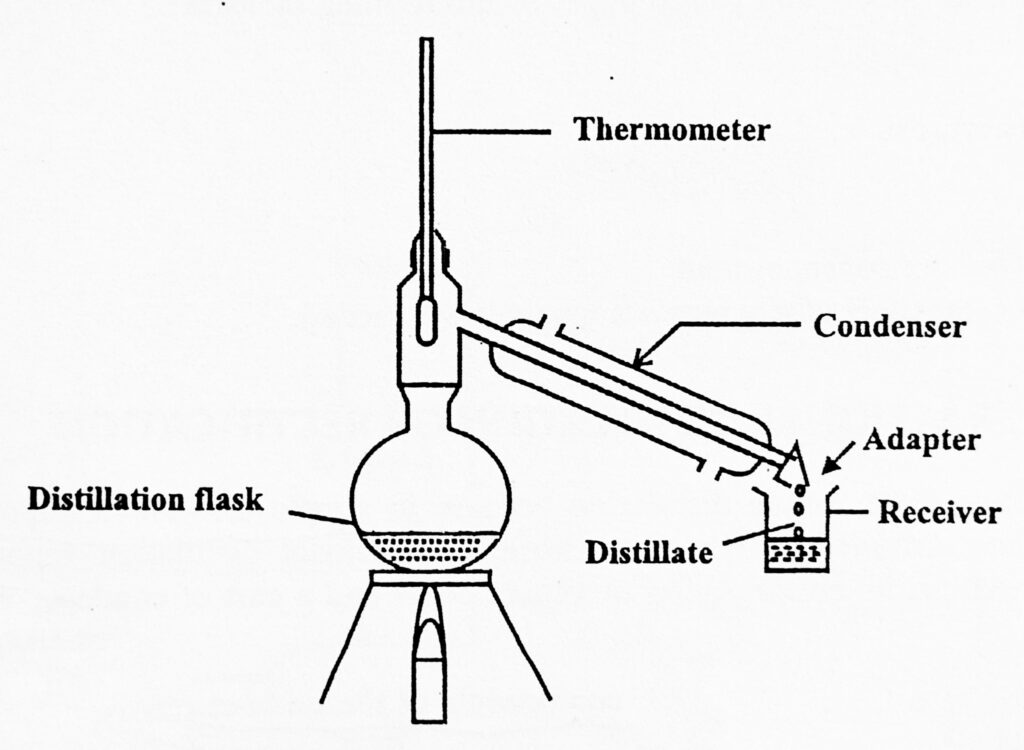
The liquid is filled into the flask up to one-half to two-thirds of its volume. There may be chances of bumping which is avoided by placing a small porcelain piece or pumice stone before distillation. The pumice stone should never be added during distillation or in superheated liquid, it may produce sudden splashing. On the laboratory scale, the water bath is maintained at a temperature below 100°C for distilling liquid with a boiling point below 80°. For inflammable liquids, the heating mantle is preferred. The liquid starts to boil after some time. At this time the temperature of the distillate is observed by the thermometer. This temperature is equal to the boiling point of the liquid. As a result, vapors start to rise up and pass through the condenser. Here vapor is condensed and liquid is collected into the receiver flask. The process is continued.
On large scale still is made of stainless steel or copper. In this case, steam is used as the heating medium. The steam is circulated into the jacket around the vessel. The thermometer is attached to the still to check the temperature of the boiling liquid. The still is attached to the condenser and to the receiver. Metal condenser such as double pipe heat exchangers or tubular heat exchangers is used on large scale.
Applications of simple distillation in pharmacy
- This is used for the preparation of distilled water and water for injection.
- This process is used to prepare many volatile oils and aromatic water. For example Spirit nitrous ether and Aromatic Spirit of Ammonia.
- This process is also used to separate non-volatile solids from volatile liquids such as alcohol and ether.
Make sure you also check our other amazing Article on : Mechanism of Heat flow