Synthetic Evidence of Benzene
(1) Benzene is unreactive to addition reactions. Due to the presence of 03 alternate double bonds in benzene structure, the chemists of that time believe it to react easily with bromine in the dark at room temperature. But due to uniform delocalized electrons (aromaticity) between all C – C bonds, benzene regained unusual stability and does not react with bromine.
(2) The hydrogenation across double bond easily occurs.
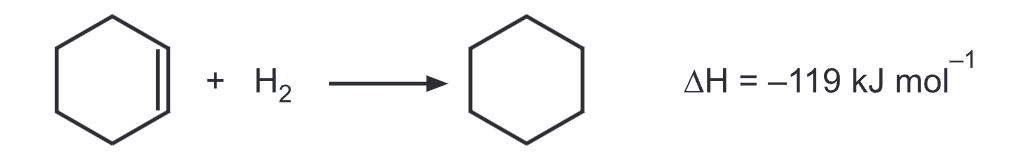
Benzene contains 03 double bonds and should liberate three times the energy that of cyclohexene. i.e., (3x 119) = 357 kJ mol¹. But the actual value for benzene is -208 kJ mol¹. So benzene is (357-119) = 152 kJ mol¹ more stable than expected as it does not contain three ordinary alternate double bonds. All these three double bonds are resonating within the ring.
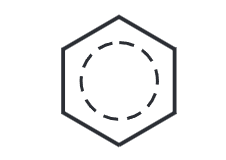
The benzene ring has thus delocalized electrons. Hence, all C-C bond lengths are the same.
Summary:
- Benzene is a flat molecule with six carbons bonded in a planar ring. It has unusual stability.
- Each carbon is covalently joined to two adjoining carbons and one hydrogen. The remaining outer electron of each carbon is shared with the electron of the next carbon in the ring. Thus, the outer electrons of all the six carbons are delocalized above and below the plane of the ring, giving the ring unusual stability.
- The six carbon atoms form a perfectly regular hexagon. There are no distinct single or double bonds within the benzene. The delocalization of the ring makes each count as one and a half bonds between the carbons. Hence, all the carbon-carbon bonds have exactly the same length-somewhere between single and double bonds.
- Benzene resists addition reactions because these reactions would involve breaking the delocalization and losing stability.
Make sure you also check our other amazing Article on : Orbital Picture of Benzene