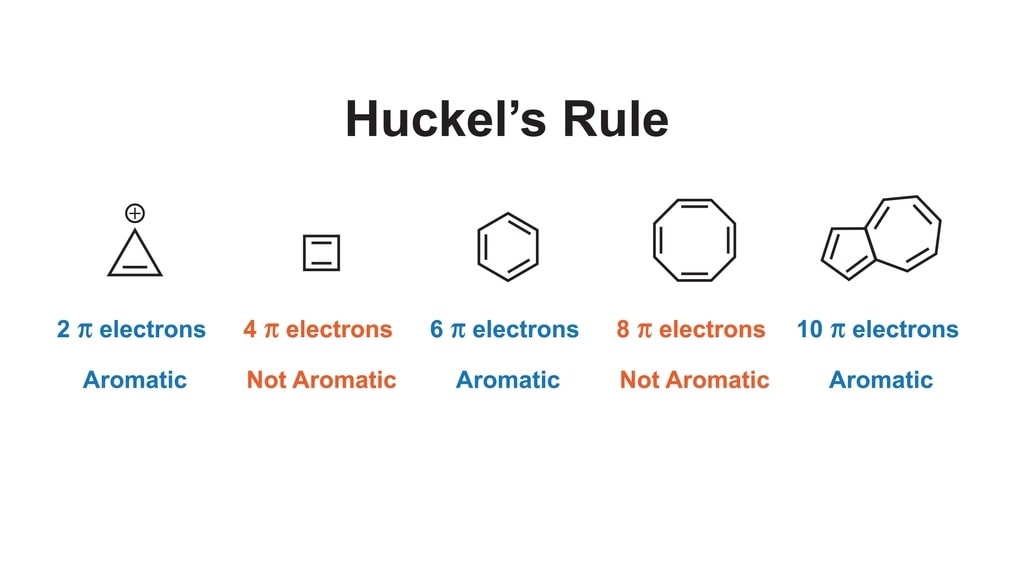
Huckel’s Rule: Aromaticity is used to describe a cyclic, planar molecule with a ring of resonance bonds that impart more stability to the molecule. Aromaticity thus describes the extra stability of planar, cyclic conjugated molecules.
In 1931, German chemist Erich Huckel proposed a rule to define the extra stability or aromaticity or aromatic properties. To possess aromaticity, the molecule
- Should be a polyunsaturated cyclic hydrocarbon,
- Should be planar,
- Should be fully conjugated, (i.e., all atoms in the ring must be able to participate in pi bonding through resonance.)
- Should have 4n + 2n electrons (n >= 0)
л electrons refer to electrons with bonds or lone pairs within p-orbitals.
For example, benzene has three double bonds. Each double bond ( bond) contains 2pi electrons. So benzene has 6pi electrons i.e., For benzene,
For benzene, n = 1 which is a positive value. Hence, benzene is aromatic. Huckel’s rule is also applicable to anions like cyclopentadienyl anion (C5H5 –) with six π electrons. Because of its aromaticity, the anion is more stable than the neutral cyclopentadienyl radical.
4n + 2= 6
4n = 6-2 = 4
n = 1
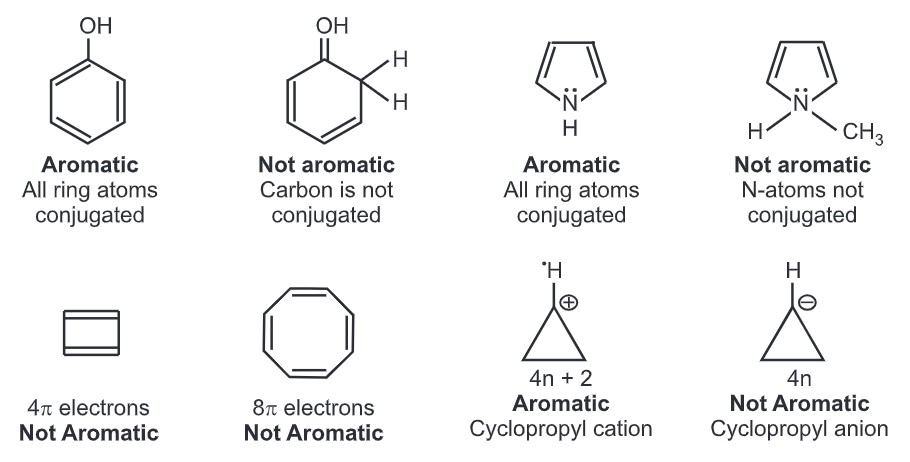
Polycyclic molecules like pyrene (16 pi electrons) and coronene (24pi electrons) although aromatic in nature, fail to follow Huckel’s rule. Only monocyclic systems seem to follow Huckel’s rule.
To become fully conjugated, an atom in the ring should not be attached to four bonds. e.g. sp3 carbon or nitrogen.
Heterocyclic compounds consist of hetero atoms like N, O, and S. A large number of heterocyclic compounds follow Huckel’s rule of aromaticity e.g.,
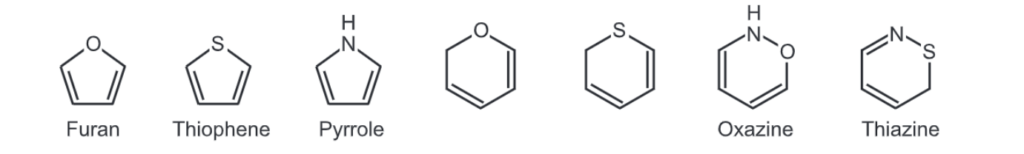
The lone pairs of electrons present on these hetero atoms can participate in resonance with electrons of the ring that make these heterocyclic molecules aromatic.
Make sure you also check our other amazing Article on : Resonance of Benzene