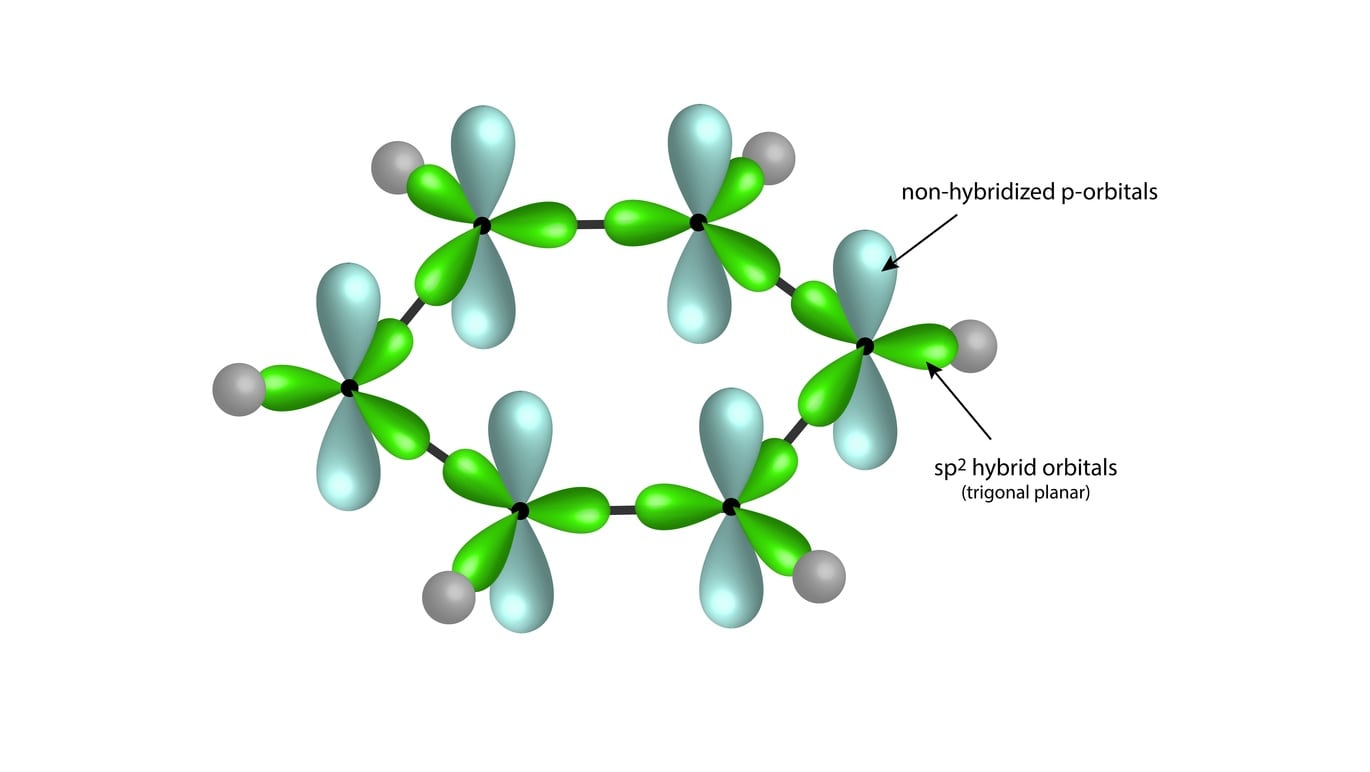
Orbital Picture of Benzene: The delocalization of the p-orbital carbons on the sp2 hybridized carbons leads to the aromaticity of benzene. There are a total of six p-orbital electrons that form the stabilizing electron clouds above and below the aromatic ring.
Benzene has a planar hexagonal structure in which all the carbon atoms are sp2 hybridized and all the C-C bonds are equal in length.
Each carbon has an unhybridized p-orbital perpendicular to the plane of the ring. It makes up the conjugated x-system.
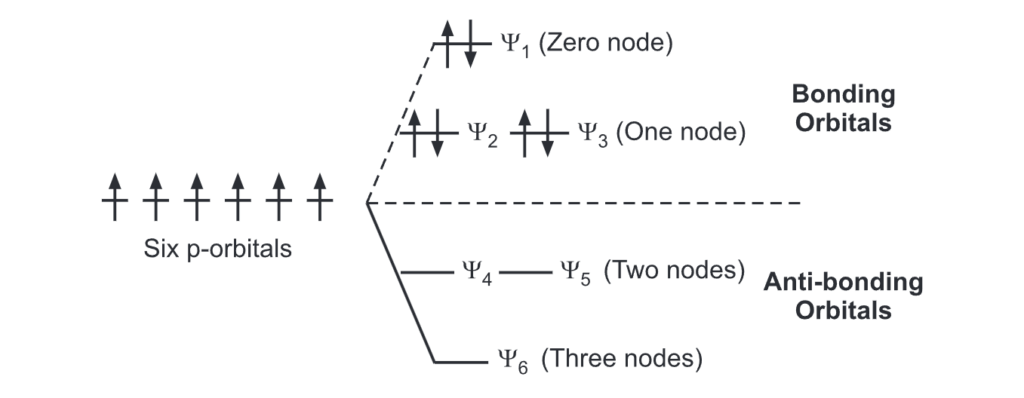
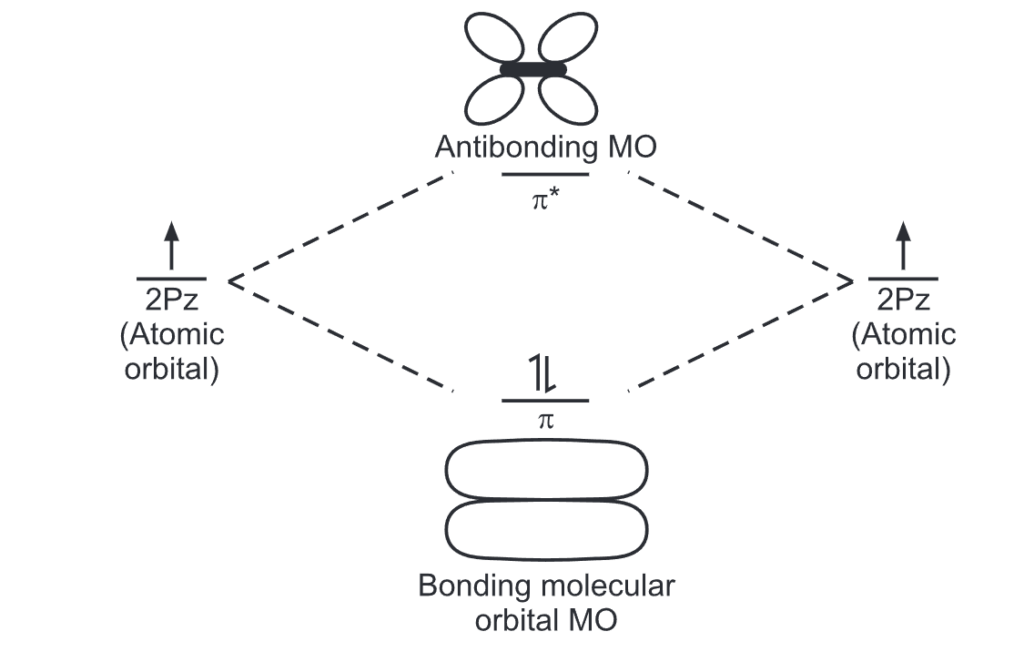
The p-orbitals (one on each carbon) linearly overlap to generate six molecular orbitals, three bonding, and three antibonding. It is this completely filled set of bonding orbitals or closed shell that gives the benzene ring its thermodynamic and chemical stability.
Electrons that spend most of their time between the nuclei of two atoms are placed into the bonding orbitals, and electrons that spend most of their time outside the nuclei of two atoms are placed into antibonding orbitals. The bonding orbitals are at lower energy than the antibonding orbitals. So they are the first to fill up.
Make sure you also check our other amazing Article on : Reactions of Cyclopropane and Cyclobutane