Usually, two kinds of elimination kinetics are involved in drug elimination: zero-order and first-order. In zero-order elimination kinetics, the elimination of a constant quantity of the drug per unit of time is eliminated from the organism. Thus, it is not dependent on the concentration of drug in the blood; hence called zero-order kinetics. In the case of first-order elimination kinetics, the elimination of a constant fraction of the drug quantity present in the organism is eliminated per unit of time. Thus, the elimination is dependent on the concentration of the drug; hence it is called as first-order kinetics.
Zero-order elimination kinetics
If a graph of drug concentration versus time is plotted, the profile during the elimination phase is linear in nature.
First-order elimination kinetics
If the amount of drug A is decreasing at a rate which is proportional to the concentration of A, the amount of drug A remaining in the body, then the rate of elimination of drug A can be described as:
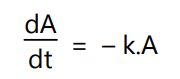
where, k = The first order rate constant.
In the case of first-order reaction, the reaction proceeds at a rate that is dependent on the concentration of A present in the body. It is assumed that the process of ADME follows first-order reactions and most drugs are eliminated in this manner.
Most drugs used in clinical practice at therapeutic doses show the first-order process; i.e. the rate of elimination is dependent on the concentration of drug remaining in the body. However, there are notable exceptions; e.g. phenytoin and high-dose salicylates. For drugs that show a first-order elimination process, it can be shown that, as the amount of drug administered increases, the body will eliminate the drug at a higher rate and the drug may not accumulate in the body. However, if you continue to increase the amount of administered drug then all drugs will change from showing the first-order process to a zero-order process, e.g. in an overdose situation.
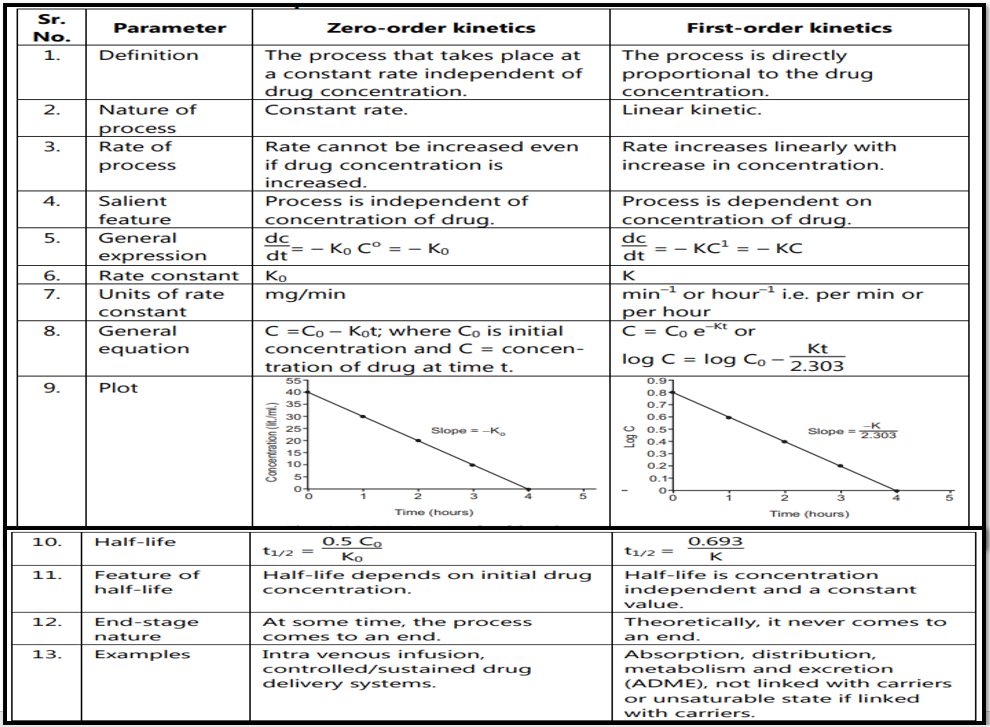
The difference between zero-order kinetics and first-order kinetics is depicted in table 1.1.
Table 1.1: Comparison between zero-order and first-order kinetics
Make sure you also check our other amazing Article on: Enzyme Induction and Inhibition
