The term alkaloid was introduced by W. Meissener at the beginning of the 19th century to designate natural substances reacting like base, in other words like alkalis (from the Arabic alkali = soda and from the Greek word eidos = appearance).
The definition of alkaloids is not simple and precise and sometimes it is difficult to distinguish the thin line between alkaloids and other natural nitrogen-containing metabolites.
Natural nitrogen-containing compounds are:
- Amino acids
- Amines
- Alkaloids
- Indole
- Purines, pyrimidines, and cytokinins
- Cyanogenetic glycosides
- Chlorophylls
An alkaloid is an organic compound of natural origin which contains a nitrogen atom which is more or less basic, is of limited distribution, and has at low doses marked pharmacological properties.
This grouping has a sound basis which is confirmed by the fact that these compounds have in common some reactions of precipitation with the general reagent for alkaloids. Their nitrogen atom is a part of the heterocyclic system and causes significant pharmacological activity.
According to some authors, they occur only in the vegetable kingdom. They are found as salt and it can also say that they are formed biosynthetically from an amino acid.
Pseudo alkaloids most often have all of the characteristics of the true alkaloid but they are not derived from amino acids. Some of the most known examples are Isoprenoid, Aconite, Aconine, Hypoaconitine, etc.
Steroidal alkaloids like Conessine, Solanidine are a few examples. They are also known as heterocyclic nitrogen-containing substances arising from the metabolism of Acetate. An example is a coniine, the toxic principle of Hemlock.
Proto alkaloids are simple amines in which the nitrogen atom is not part of a heterocyclic ring. They are basic in nature and derived from amino acids. A few examples of these are Colchicine, Ephedrine, and Pseudoephedrine.
Table of Contents
DISTRIBUTION
Alkaloids occur exceptionally in bacteria (Pseudomonas aeruginosa) and rather rarely in fungi (Psilocin from the hallucinogenic mushroom).
The Pteridophyta rarely contains alkaloids. Thus alkaloids are compounds essentially found in the angiosperm.
Alkaloid concentrations have a wide range of variation from a few ppm (for example Catharanthus roseus contain 3 gm alkaloid in one metric ton leaves) to more than 15% in the bark of the trunk of Cinchona ledgeriana.
Sometimes they do contain only one constituent (for example Hyoscyamine from the leaves of belladonna) but must often they yield a complex mixture which may be dominated by one major constituent. As a general rule, all of the alkaloids of a given plant have a common biogenetic origin even if their structure may at first be seen quite different.
The conc. of alkaloids can vary from part to part and some parts may contain none. For example, Quinine accumulates in the trunk bark of cinchona but is completely absent from leaves. For a long time alkaloids used to be considered products of the metabolism of the plant only. In fact, alkaloids also occur in animals (Arthropods who secrete them in very small quantities in their exocrine glands).
Sometimes they are volatile enough to act as chemical signals defense compounds known as allomones or communication compounds called pheromones.
Localization
In the plant, alkaloid occurs as a soluble salt (citrate, Malate, tartrate, malonates, benzoates, isobutyrates) or in combination with tannins.
They are often localized in the peripheral tissue external layer of the bark and root or seed tegument. Alkaloids synthesis takes place at specific sites (growing root, chloroplast, laticiferous cell).
FUNCTIONS OF ALKALOIDS
Alkaloids are poisonous in nature but when used in small quantities they exert useful physiological effects on animals and human beings.
Their exact role in the plant is still a topic of research. Some of the predicted roles are:
- They are reserve substances which can supply nitrogen.
- They might be the defensive mechanism for plant growth in the dry region to protect from grazing animals, herbivores, and insects.
- They may be the end product of detoxification mechanism in plant and by this way check formation of substance which may be prove to harmful to the plant.
- The possible role as a growth regulatory factor in the plant.
- They are present normally in conjugation with plant acid-like mercuric acid, cinchotannic acid, etc. Therefore alkaloids could be acting as carriers within the plant for the transportation of such acids.
PHYSICOCHEMICAL PROPERTIES
- Alkaloids have a molecular weight of 100-900.
- Although most of the bases that do not contain oxygen atoms are liquid at ordinary temperature (examples: nicotine, coniine). Those that do contain oxygen atoms are normally crystallized solids and in rare cases, they are colored compounds (for example berberine).
- Almost all of the crystallized bases rotate the plane of polarized light and have melting points without decomposition especially below 200o C.
- As a general rule alkaloids as bases are not soluble in water. They are soluble in polar or slightly polar organic solvents.
- The basicity of alkaloids varies greatly. Since this property depends entirely on the availability of the lone pair of electrons on the nitrogen atom.
- The electron-withdrawing group in close proximity to the nitrogen atom decreases the basicity, whereas electron-donating group enhances it.
- The basic character allows the formation of salts with mineral acids (i.e. hydrochloride, sulphates, and nitrates) and organic acids (i.e. tartrate, sulfonate).
- Alkaloidal salts are generally soluble in water and in dilute alcohols and they are except in rare cases not soluble in the organic solvent.
- Pseudo alkaloids and proto alkaloids show higher solubility in the water while free bases of alkaloids are soluble in the organic nonpolar immiscible solvent.
DETECTION AND CHARACTERIZATION
The different reagents used for the detection of alkaloids are:
- Mayer reagent (Potassium mercuric iodide solution) gives cream-colored precipitate.
- Dragendorff reagent (Potassium bismuth iodide solution) shows a reddish brown precipitate.
- Wagner reagent (Iodine Potassium Iodide solution) yields reddish-brown precipitate.
- Hager’s reagent (saturated Picric acid solution) gives yellow color precipitate.
- ρ-dimethyl amino benzaldehyde uses for ergot alkaloids and pyrrolizidine alkaloids.
- Cerium and ammonium sulfate for different indole (yellow), dihydro indole (red), and β-amino acelates (blue).
- Caffeine, purine derivatives do not precipitate like most alkaloids. It is detected by mixing with a very small amount of potassium chlorate and a drop of HCl, evaporating to dryness and exposing the residue to ammonia vapor. Purple color is produced with caffeine and other purine derivatives (Murexide test).
Care must be taken in the application of these alkaloidal tests, as the reagent also gives a precipitate with proteins. During the extraction of alkaloids from the plant and subsequent evaporation, some proteins will not be extracted and others will be made insoluble (denatured) by the evaporation process and may be filtered out. If the original extract has been concentrated to low bulk and the alkaloids extracted from an alkaline solution by means of an organic solvent and then transferred into dilute acid (e.g. Tartaric acid) the latter solution should be protein-free and ready to test for alkaloids.
Classification of Alkaloids
The classification is mainly based on pharmacological activity, taxonomical distribution, their biogenetic origination, and the presence of chemical entities.
Pharmacological Classification
Depending on the physiological response the alkaloids are classified under various pharmacological categories like CNS stimulants or depressants, sympathomimetics, analgesics, purgatives, etc. The main drawback of this system is that it does not take into consideration about chemical nature of the crude drug. Within the same drug, the individual alkaloids may exhibit different actions. Example-
- Morphine is narcotic and analgesia while codeine is mainly antitussive.
- Cinchona quinine is antimalarial whereas quinidine is a cardiac depressant.
Taxonomic Classification
This method classifies the vast number of alkaloids based on their distribution in various plant families like solanaceous alkaloids in the Solanaceae family or papilionaceous alkaloids in papillionaceae family. The grouping of alkaloids is done as per the name of the genus in which they occur e.g. Ephedra, cinchona, etc. The chemotaxonomic classification has been further derived from this classification.
Biosynthetic Classification
This method gives the significance to the precursor from which the alkaloids are biosynthesized in the plant. Hence the variety of alkaloids with different taxonomic distribution and physiological activities can be brought under some group if they are derived from the same precursor i.e. all indole alkaloids from tryptophan are grouped together. Alkaloidal drugs are categorized on the fact whether they are derived from amino acids precursor as ornithine, lysine, phenylalanine, tryptophan, etc.
Chemical Classification
This is the most accepted way of classification of alkaloids which basically depends on the ring structure present in the alkaloid. The alkaloidal drugs are broadly categorized into two divisions:
- True alkaloids (subdivided into 12 groups).
- Proto alkaloids or biological amines and pseudo alkaloids.
True alkaloids
- Pyrrole and pyrrolidine: Hygrine, coca species.
- Pyridine and piperidine: Arecoline, Anabasine, coniine, trigonelline.
- Pyrrolizidine: Echimidine, symphitine.
- Tropane (piperidine/N-methyl pyrrolidine): Atropine, hyoscine.
- Quinoline: Quinine, Quinidine, Chinchonine.
- Isoquinoline: Morphine, codeine.
- Aporphine (reduced isoquinoline – Naphthalene): boldine.
- Indole (Benzpyrole): Vincristine, Ergometrine, Reserpine.
- Imidazole: Pilocarpine, Isopilocarpine.
- Norlupiname: Cytisine, Spartine.
- Purine (pyrimidine/ imidazole): Caffine/ theofronine, theophylline.
- Steroidal (cyclo pentano per hydro phenanthrene ring): Solanidine, Conessine.
Pseudo alkaloids
Diterpenes- Aconitine, Aconine
Proto alkaloids
Alkylamines (amino alkaloids) – Ephedrine, colchicine
VINCA
Synonyms: Catharanthus, Periwinkle
Biological source: It consists of dried whole plant of Catharanthus roseus L or Vinca rosea
Family: Apocynaceae.
Geographical source: It is indigenous to Madagascar and cultivated in South Africa, India, the USA, Europe, and Australia
Cultivation and Collection
It grows well at an altitude up to 500 mt. This plant is found all over India but grown well in tropical and subtropical areas (like southern and northeastern parts) of India. It does not require any particular type of soil. It grows well in light sandy soil which should be rich in humus content. The 100 cm rainfall is most preferable for its cultivation. The fresh seeds are used for its propagation. The seeds should be shown in the nursery or direct sowing can also be done. The seeds are mixed with sand (1:10) and sown in rows having a 45 cm distance in between two rows. The sowing should be in monsoon season. The plants are thinned out upon sufficient growth. In other cases, nursery sowing is more economical. They are sown in the nursery in February or March season and transplanted in an open field after 2 months or when they achieve 6-7 cm height. The plants are drought resistant and do not require much water supply. The plants also do not require any type of fertilizers. A mixture of nitrogen, phosphorus, and potassium gives better results. Farmyard manures are used and weeding is done from time to time periodically. The stems are cut after 1 year of growth. The leaves, stems, and seeds are separated and dried in the air. The roots are collected by the digging out method followed by ploughing. The roots are washed properly, shade dried, and finally packed into bales. The yield is about 1 to 5 tons per hectare (roots), 1 to 2 tons per hectare (stem), and 3 to 4 tons per hectare (leaves).

Macroscopical characters:
Colour: Green (Leaves), Pale grey (Roots), Purple or pinkish white or caramine red (Flowers)
Odor: Characteristic
Taste: Bitter
Other features: The plant is an erect, pubescent herb having branched taproots.
Leaves: Simple, petiolate, ovate, or oblong and glossy.
Flowers: Bracteate, pedicellate, complete, and hermaphrodite.
Fruits: Follicles with many black seeds.
Constituents:
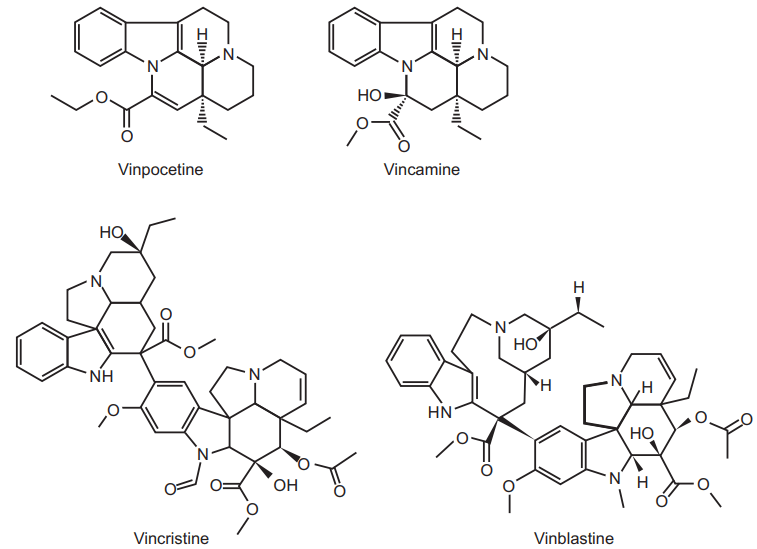
- About 150 alkaloids have now been isolated from Catharanthus roseus e.g. ajmalicine, serpentine, tetrahydrolalstonin and lochnerine, etc.
- The plant contains a large number of indole alkaloids, out of them about 20 dimeric indole dihydroindole alkaloids contains antineoplastic activity including vincristine and vinblastine. These two alkaloids are much significant.
- Vinblastine is produced by coupling of the indole alkaloid catharanthine (indole alkaloid part) and vindoline (dihydro indole alkaloid part).
- About 500kg of the drug gives 1 gm of vincristine. Vincristine concentration is very low in plant extract (about 0.0002 percent) which makes it very costly. So attempts are made for its synthesis.
- The plant contains alkaloids in very low concentration; by tissue culture technique its production can be increased.
Uses:
Vinca plant is used to extract alkaloids like vincristine, vinblastine, and ajmalicine. Vincristine sulphate acts on mitotic cell division of metaphase and arrests the cell for further division; hence used as an antineoplastic drug whereas vinblastine sulphate acts on mitosis of metaphase and interferes in amino acid metabolism. It suppresses the immunity and uses in Hodgkin’s disease, lymphoma, and choriocarcinoma treatment. Vincristine is applied by intravenous route of administration in leukemia, Hodgkin’s disease, sarcoma of reticulum cells, lymphosarcoma, and myosarcoma treatment. Vinca alkaloids are also used in diabetes and high blood pressure treatment. The maximum dose of vincristine sulphate is up to 2 mg but 10 to 30 µg/kg body weight administered intravenously whereas the dose of vinblastine sulphate is about 100 µg/kg body weight administered intravenously.
Other species: Catharanthus lengifolius, C. Trichophyllus.
Make sure you also check our other amazing Article on : Extraction of Alkaloids
