(i) Acid Value:
It is defined as the number of milligrams of KOH required to neutralize the free fatty acids present in one gram of fat. Sodium hydroxide may also be used. Phenolphthalein is used as an indicator. It is a relative measure of rancidity as free fatty acids are normally formed during the decomposition of glycerides during storage. Other decomposition products include peroxides, low molecular weight aldehydes, and low molecular weight ketones. This results in a foul smell and odor (rancidity) and affects the quality of fats. This value is calculated only for animal and vegetable oils and fats. It is not applicable to waxes.
The acid value is calculated using the following equation
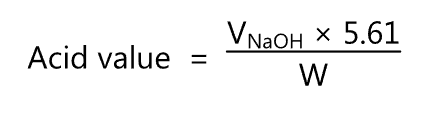
where,
VNaoH = Volume of sodium hydroxide titrant (ml) used.
W = Weight of the fatty oil (g) being examined.
The acid value in a given sample of fat/oil should not be more than the value specified in the individual monograph.
In the case of a colorless sample, phenolphthalein is an indicator of choice. In the case of strong-colored samples (e.g., palm oil sometimes has a strong orange color), it is difficult to identify the end point of titration using phenolphthalein. In such a case, bromothymol blue may be used to give a color change from orange or yellow (in acidic solution) to a darker greenish color (in neutral condition). It slowly turns blue in alkaline conditions.
(ii) Saponification Value:
Saponification literally means “Soap making”. This is done by warming a known amount of the fat with a strong aqueous caustic soda solution, which converts the free fatty acid present in the fat into a soap (which is a salt). This soap is then removed and the amount of fat remaining is then determined.
It is a measure of the totally free and combined acids, especially in a fat, wax, or resin expressed as the number of mg of KOH required for the complete saponification (to form soap) of one gram of sample.
The long chain fatty acids have a relatively fewer number of carboxylic functional groups per unit mass of the fat as compared to short chain fatty acids. Hence long chains have a low saponification value.
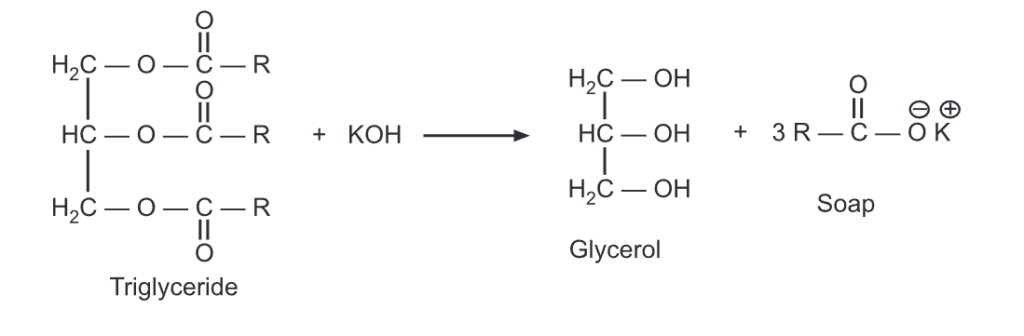
Procedure: The sample is first saponified by adding 0.5 mol/L ethanolic potassium hydroxide solution and then the excessive KOH is neutralized with 0.5 mol/L HCl.
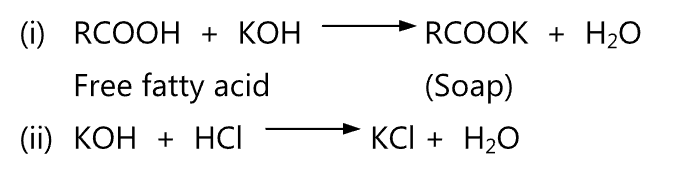
Formula:
Saponification value (mg/g) = (B-T) x TF x CI X KI/size,
where,
i) B = Blank level (25.029 ml)
ii) T = Titration volume (ml)
iii) TF = Reagent factor HCl (1.006)
iv) CI= Concentration conversion coefficient for KOH in equivalence (56.11 0.5 28.05 mg/ml)
v) KI = Unit conversion coefficient (1)
vi) Size; sample size.
Significance of saponification value:
(a) It indicates the length of the carbon chain of the acid present in the sample. The higher the value, the greater the percentage of short chain acids.
(b) It gives an idea about the average molecular weight of the fat or oil. The higher the value, the lower will be the molecular weight of the fat.
(iii) Ester value:
The ester value is the number of mg of potassium hydroxide required to saponify the esters in 1.0 g of the sample.
Initially, the sample is saponified. It is then hydrolyzed to alcohol and free fatty acids using an excess of standard KOH solution. The excess alkali is back titrated. The ester value is calculated by
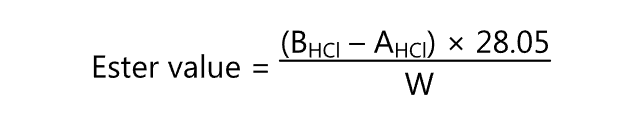
where,
BHCl = Volume (ml) of HCl consumed by the blank
AHCl = Volume (ml) of HCl consumed by the actual test, and
W = Weight (g) of the sample taken.
The ester value is calculated by subtracting the acid value of oil from the saponification value of the same oil. It is more often used to categorize waxes.
(iv) Iodine value:
The iodine value is an important analytical characteristic of oils and fats. It measures the degree of unsaturation of oil, fat, or wax. The greater the iodine value, the more will be unsaturation and the higher the susceptibility to oxidative rancidity.
The higher the unsaturation, the greater the possibility of the oils going rancid.
The quantity of thiosulfate required for blank minus the quantity required for the sample gives thiosulphate equivalent of iodide absorbed by the fat or oil sample.
The bracketed figures in the following sequence are the Iodine value of respective oil, arranged as per increasing order of saturation.
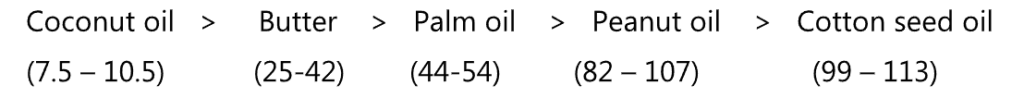
Iodine value is defined as the amount of iodine in grams that is taken up by 100 g of the oil, fat, or wax. The determination is carried out by dissolving a weighed sample in a non-polar solvent such as cyclohexane, then adding glacial acetic acid. The double bonds react with an excess of a solution of iodine monobromide in glacial acetic acid. (Hanus method) Mercuric ions are added to speed up the reaction. In the end, the excess iodine monobromide is decomposed to iodine by the addition of an aqueous potassium iodide solution, which is then titrated with a standard sodium thiosulphate solution.
Calculation: iodine value (IV) is calculated by using the following formula.
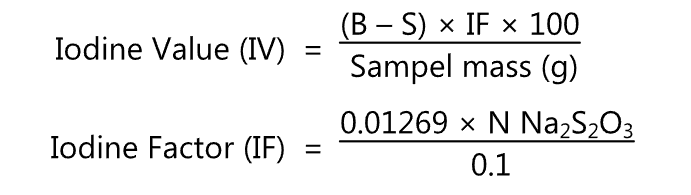
N = Normality of thiosulphate solution
B = ml thiosulphate for blank
S ml thiosulphate for sample
(v) Acetyl Value:
The acetyl value is the amount of potassium hydroxide (mg) required to neutralize the acetic acid liberated by the hydrolysis of 1 g of the acetylated substance. It thus measures the free hydroxyl groups in the given sample of fat or oil. For example, the acetyl value of castor oil (Resin oil) = is 150 because it is rich in ricinoleic acid. While the oils containing no hydroxyl fatty acids have acetyl values in the range of 5-15.
The acetyl value is calculated by
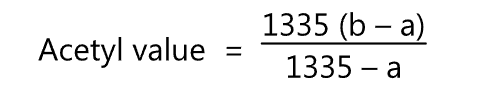
where,
- Saponification value of the sample; and
- Saponification value of the acetylated sample.
(vi) Reichert Meissl (RM) Value:
During storage, the oils and the fats undergo physical and chemical changes with the accumulation of primary and secondary oxidation products. The Reichert Meissl value indicates the amount of soluble volatile fatty acids present in a given sample. It is the number of ml of 0.1 N aqueous alkali solution necessary for the neutralization of the water-soluble volatile fatty acids distilled and filtered from 5 g of a given saponified fat.
Procedure: Pour 100 ml of the filtrate containing the soluble volatile acids into a titration flask. Add 0.1 ml of phenolphthalein indicator and titrate with the 0.1 N NaOH solution until the liquid becomes pink. The RM value can be calculated by
Reichert Meissl Value = 1.10 (T1 – T2)
where,
T1 = Volume in ml of 0.1 N NaOH solution used for the sample.
T2 = Volume in ml of 0.1 N NaOH solution used for blank.
RM value is substantially a measure of the lowest fatty acids of ghee namely butyric and caproic acids. The RM value is no longer sufficient to trace the adulteration of butter.
Make sure you also check our other amazing Article on : Difference Between Fats and Oils
