Fats and Oils Chemistry
Triglycerides are the esters of three fatty acid chains and the alcohol, glycerol.
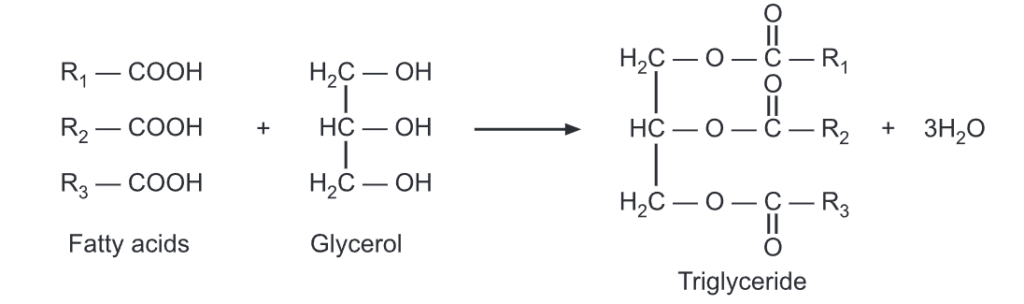
If the glycerol is esterified at all -OH sites by the same fatty acid (ie., R1 = R2 = R3), the resulting ester is called a simple triglyceride. While if glycerol is esterified by two or three different fatty acids, the resulting ester is called a mixed triglyceride. A triglyceride existing in a solid state at 250C is called fat while that exists in the liquid state at 250C is called oil.
Naturally occurring fats and oils are complex mixtures of mixed triglycerides. Fats contain triglycerides with a long and saturated fatty acid chain and are usually obtained from animal sources. Oils contain triglycerides with a short and unsaturated fatty acids chain and are usually obtained from plant origin.
Difference between fats and oils
| SR.No | Fat | Oil |
| 1. | Colourless, odourless and tasteless. | Colourless, odourless and tasteless. |
| 2. | Solid at room temperature. | Liquid at room temperature. |
| 3. | Animal origin. | Plant and fish origin. |
| 4. | Long and saturated fatty acid chain. | Short and unsaturated fatty acid chain. |
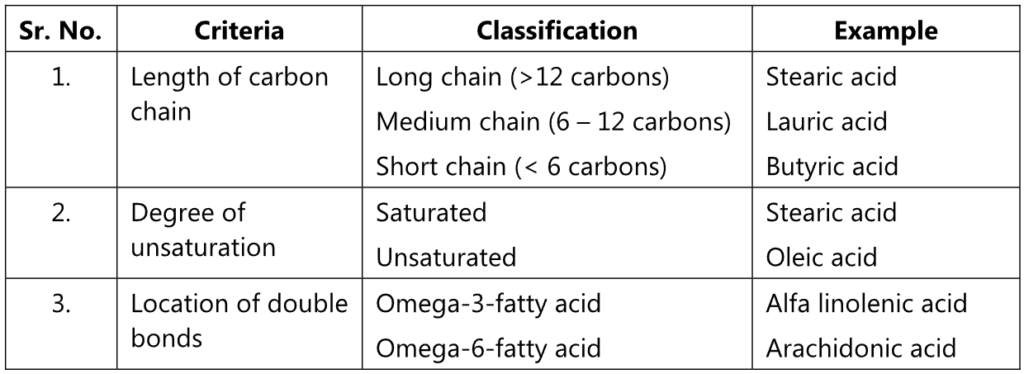
The fatty acid present in the triglycerides can be classified as:
Chemical Reaction of Fats and Oils
Hydrolysis of fats and oils
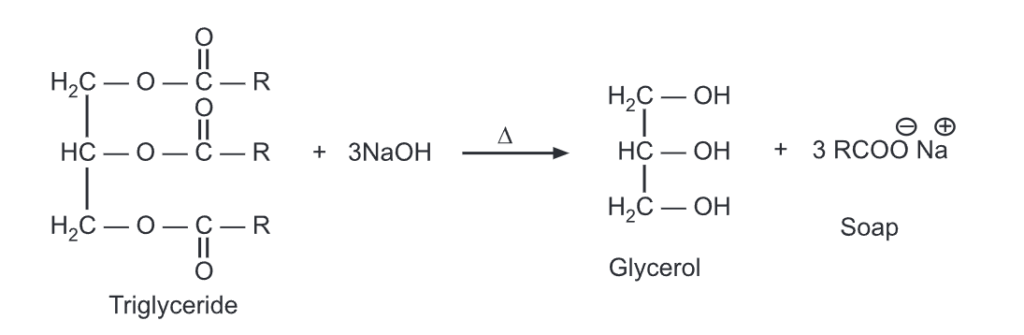
Triglycerides undergo hydrolysis under the condition of acid digestion or saponification.
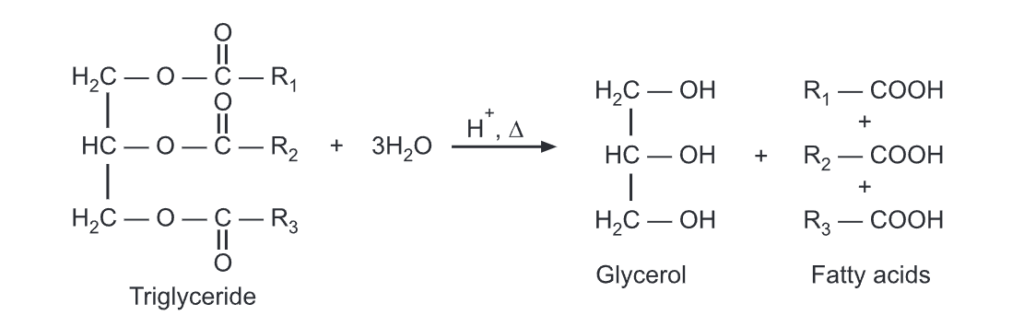
The triglyceride will be transformed into glycerol and fatty acid with step-by-step hydrolysis of each of the three ester linkages. It can be carried out in the presence of acid and heat or with suitable lipase enzymes under biological conditions.
When these fatty acids are neutralized with a base, they produce carboxylate ions which are used as soaps. The hydrolysis and neutralization can be carried out simultaneously using a base like NaOH. It produces soap in a one-step reaction called saponification.
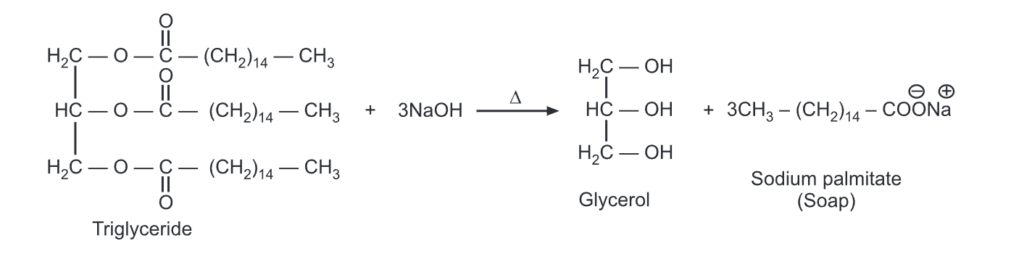
For example,
Hydrogenation (Reduction) of fats and oils
It converts unsaturated compounds (oils) into saturation derivatives (fats) in presence of a catalyst such as nickel, palladium or platinum. Hydrogenation is typically carried out by bubbling H2 gas through the heated oil, in the presence of a metal catalyst. Oils are usually only partially hydrogenated, so that the product is not completely saturated, giving a semisolid fat.
In absence of a catalyst, a very high temperature is usually required to carry out hydrogenation. Hydrogenation is carried out at different temperatures and pressures depending upon the degree of unsaturation in the substrate and the activity of the catalyst. Natural unsaturated fatty acids have cis double bonds. The catalysts and reaction conditions used for hydrogenation reactions can also lead to isomerization at the site of unsaturation (i.e., from cis to trans) across the double bond.
Saponification of fats and oils
When a triglyceride is hydrolyzed with a strong base like NaOH, Soap (fatty acid sodium salts) is formed. Saturated fats upon NaOH hydrolysis produce hard soaps while KOH is used with oils (unsaturated fats) to produce soft/liquid soaps.
Rancidity of fats and oils
When triglycerides containing short chain fatty acids (like butyric acid, and pentanoic acid), are hydrolyzed, the carboxylic acid products are foul-smelling and foul-tasting (i.e. rancid). Air oxidation of fatty acids may sometimes cleave the double bonds to form short chain carboxylic acids. These oxidation products are foul tasting and smell horrible. Rancidity may also develop due to bacterial hydrolysis of triglycerides.
Drying of oils
It is used to determine the moisture content of the oil. The moisture content of oils and fats is the loss in mass of the sample on heating at 105-1100C in a hot air oven for 1 hr. under specified conditions. After heating the sample, it is cooled and weighed. The process is repeated until the change in the weight between two successive observations does not exceed 1 mg.
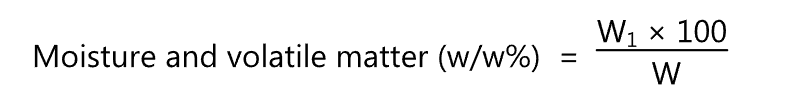
where W1 = Loss in gm of the material on drying.
W = Weight in gm of the material taken for the test.
Make sure you also check our other amazing Article on : Reactions Of Carboxylic Acid
