Beckmann rearrangement is an acid-catalyzed rearrangement of an oxime to an amide. It is named after the German Chemist, Ernst Otto Beckmann (1853-1923).
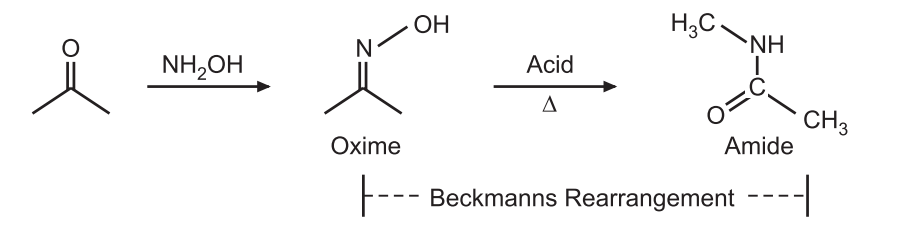
Oximes derived from ketones form amides while oximes derived from aldehydes from nitriles. e.g.,
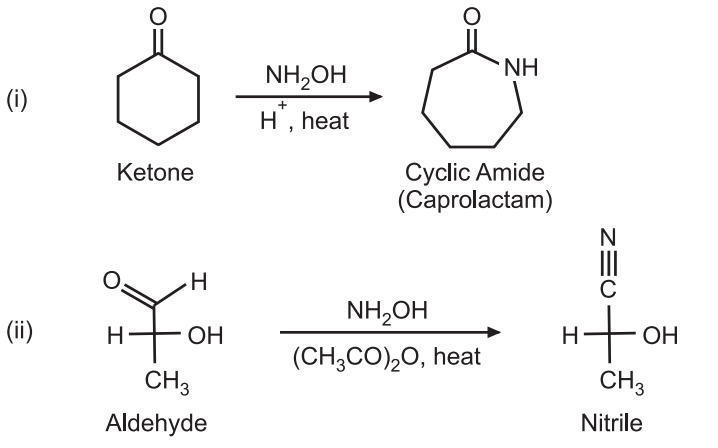
Open chain oxime gives an open-chain amide while cyclic oximes give lactam. The Beckmann rearrangement is often catalyzed by acid. The Beckmann solution, acetic acid, hydrochloric acid, and acetic anhydride is widely used to catalyze the rearrangement. Other acids like polyphosphoric acid, sulfuric acid, or phosphorous pentachloride can also be used. This solution consists of acetic acid, hydrochloric acid, and acetic anhydride and is widely used to catalyze rearrangement. Besides the acids, other catalysts like tosyl chloride, thionyl chloride, phosphorus pentachloride, sodium hydroxide, triethylamine, etc. may also be used.
Mechanism of Beckmann Rearrangement
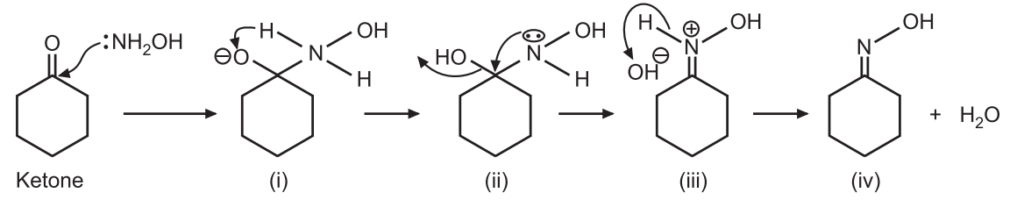
(a) Formation of an oxime: In the first step the nitrogen adds to the carbonyl carbon
- followed by proton transfer,
- then the lone pair of nitrogen displaces the hydroxide,
- and finally, oxime is generated by the deprotonation of nitrogen.
(b) Beckmann rearrangement:
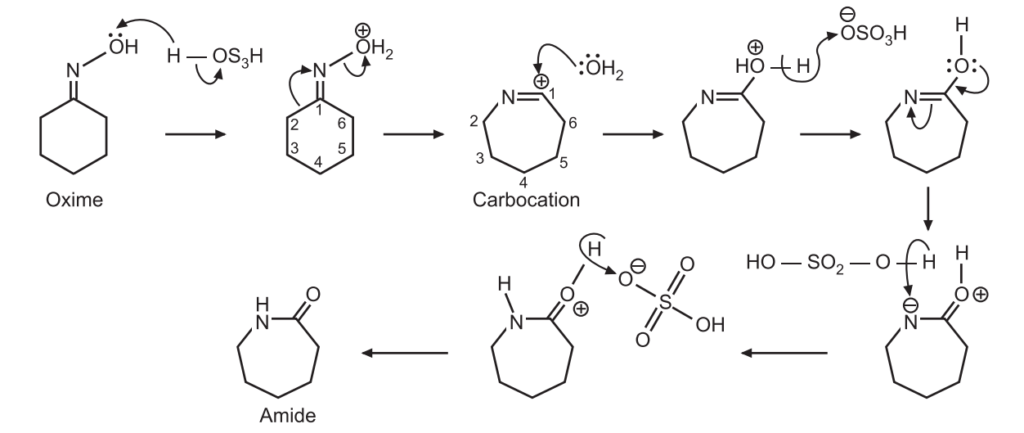
The reaction begins by protonation of the oxime oxygen group forming a better leaving group. The oxime can also be treated with acetic anhydride, (CH3CO)2 O (converting the oxime oxygen to acetate, a better leaving group than OH). The R group trans to the leaving group and then migrates to the nitrogen, resulting in a carbocation and the release of a water molecule (deprotonation).
The free carbocation is then attacked by water. The positively charged oxygen is deprotonated by the base. Protonation of nitrogen followed by deprotonation of oxygen gives the final product, Amide.
Applications in drug synthesis:
(1) One of the synthetic routes of paracetamol (an analgesic antipyretic) involves the conversion of a methyl ketone to acetanilide via a Beckmann rearrangement.
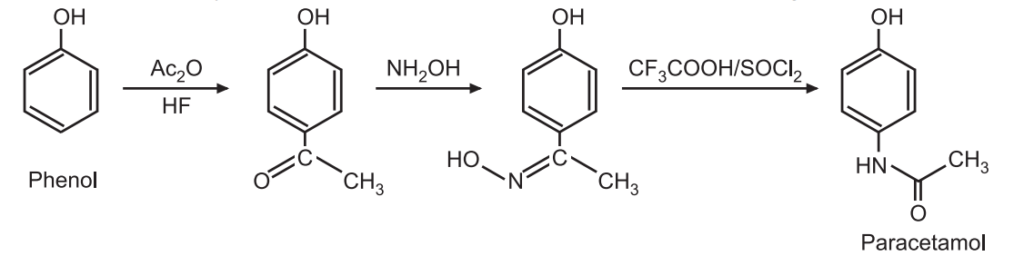
(2) This reaction is an excellent tool to prepare aza (ring containing nitrogen) derivatives of steroidal (anti-inflammatory) drugs.
Make sure you also check our other amazing Article on : Dakin Reaction