Tonicity is a measure of effective osmolarity or effective osmolality in cell biology. Osmolality and osmolarity are properties of a solution, independent of any membrane. Osmolality is a concentration scale to express the total concentration of solute particles and is directly related to any of the four colligative properties. Buffered isotonic solutions are derived from molality by factoring in the dissociation of electrolytic solutes.
Osmolality = Molecular weight × Number of particles/molecule
Tonicity is a property of a solution about a membrane and is equal to the sum of the concentrations of the solutes which have the capacity to exert an osmotic force across that membrane. Tonicity depends on solute permeability. The permeable solutes do not affect tonicity but the impermeable solutes do affect tonicity. If a semi-permeable membrane is used to separate solutions of different solute concentrations, a phenomenon known as osmosis occurs to establish concentration equilibrium. The pressure driving this movement is called osmotic pressure and is governed by the number of particles of solute in the solution. If the solute is a non-electrolyte, then the number of particles is determined solely by the solute concentration. If the solute is an electrolyte, the number of particles is governed by both the concentration and degree of dissociation of the substance.
The distinction between the isosmotic and isotonic terms comes with the realization that red blood cell membranes are not perfect semipermeable membranes but allow passage of some solutes, such as alcohol, boric acid, ammonium chloride, glycerin, ascorbic acid, lactic acid, etc. A 2% solution of boric acid when physically measured found to be isosmotic (containing the same number of particles) with blood and not isotonic (exerting equal pressure or tone) with the blood but is isotonic with tears. This differentiation is not having any great significance and therefore isotonicity values are calculated based on the number of particles in the solution is sufficient. The clinical significance of all this is to ensure that isotonic or isosmotic solutions do not damage tissue or produce pain when administered.
Buffered Isotonic Solutions are generally classified into three types; hypertonicity, hypotonicity, and isotonicity. Hypertonic, isotonic, and hypotonic solutions are defined in reference to a cell membrane by comparing the tonicity of the solution with the tonicity within the cell.
Hypertonicity
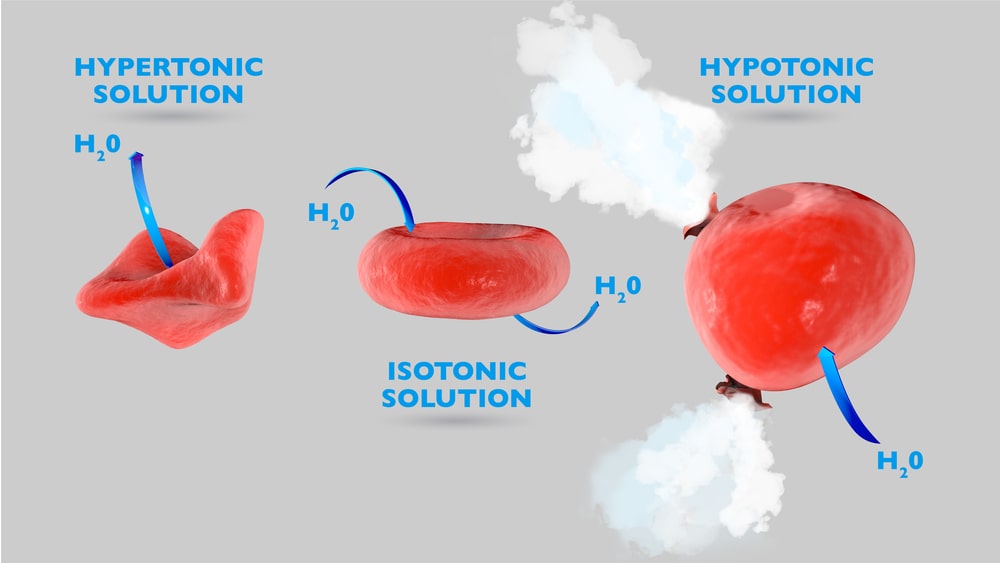
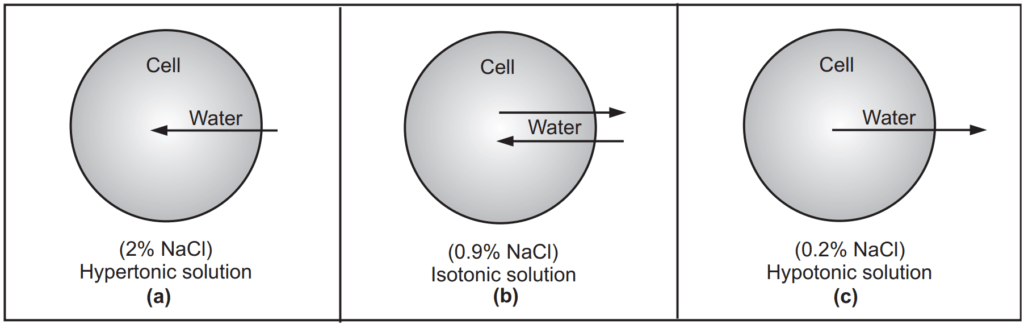
A solution having higher osmotic pressure than the body fluids (or 0.9% NaCl solution) is known as a hypertonic solution. These solutions draw water from the body tissues to dilute and establish equilibrium. An animal cell in a hypertonic environment is surrounded by a higher concentration of impermeable solute than exists inside of the cell. For example, if 2.0% NaCl solution is added to blood (defibrinated), osmotic pressure directs a net movement of water out of the cell causing it to shrink (the shape of the cell becomes distorted) and wrinkled (crenated), as water leaves the cell… This movement is continued until the concentrations of salt on both sides of the membrane are identical. Hence, 2.0% NaCl solution is hypertonic with the blood, Fig. 1.1 (a).
Isotonicity
The solution that has the same osmotic pressure as that of body fluids is said to be isotonic with the body fluid. Body fluids such as blood and tears have osmotic pressure corresponding to that of 0.9 % NaCl or 5% dextrose aqueous solution thus, a 0.9% NaCl or 5% dextrose solution is called an isosmotic or isotonic. The term isotonic means equal tone and is used interchangeably with isosmotic regarding specific body fluids. Isosmotic is a physicochemical term that compares the osmotic pressure of two liquids that may or may not be body fluids. A cell in an isotonic environment is in a state of equilibrium with its surroundings with respect to osmotic pressure. When the amount of impermeable solute is the same on the inside and outside of the cell, osmotic pressure becomes equal. When the amount of impermeable solute is not the same on the inside and outside of the cell, the force of water trying to exit or enter the cell to maintain the balance. This pressure drives hypertonic or hypotonic cells to become isotonic. For example, a 0.9% w/v solution of NaCl in water is isotonic in relation to RBCs and their semi-permeable membranes Fig. 1.1 (b).
Requirements of isotonic solutions are that they must not cause any contraction or swelling of the tissues. The product must not produce discomfort when instilled in the eye, nasal tract, blood, or other body tissue, for example, isotonic NaCl. On addition of 0.9 g NaCl/100 mL (0.9%) into blood (defibrinated), the cells retain their normal size. The isotonic solution should be restricted to solutions having equal osmotic pressures with respect to a particular membrane.
The addition of any compound to a solution affects its isotonicity, causing changes in the osmotic pressure of a solution. It should not be affected only by drugs but also by any buffer compounds added to the formulation. Therefore, it is necessary to add additional NaCl to bring the solution to isotonicity. Adjustment of isotonicity is required for several dosage forms such as parenteral preparations, for example, IV infusions, irrigating solutions; lotions for open wounds, subcutaneous injections, preparations meant for diagnostic applications, and solutions meant for intrathecal injections, nasal drops, and ophthalmic drops.
Hypotonicity
A solution with low osmotic pressure than body fluids is known as a hypotonic solution. Administration of a hypotonic solution produces shrinking of tissues (painful swelling) as water is pulled from the biological cells (tissues or blood cells) to dilute the hypertonic solution. The effects of administering a hypotonic solution are generally more severe than with hypertonic solutions since ruptured cells can never be repaired. Hypotonic solutions show the opposite effect compare to hypertonic solutions in that the net movement of water is into the cell causing them to swell. If the cell contains more impermeable solute than its surroundings, water enters it. In the case of animal cells, they get swelled until burst; but this doesn’t happen to plant cells i.e. they do not burst due to the reinforcement their cell wall provides. If 0.2% NaCl solution is added to blood (defibrinated), the cells get swelled and burst. Therefore, 0.2% NaCl solution is hypotonic with respect to the blood, Fig. 1.1 (c). A 2.0% solution of boric acid has the same osmotic pressure as blood, but it is hypotonic because boric acid passes freely through the cell membrane regardless of concentration.
Make sure you also check our other amazing Article on : Classification of Surfactants