What is centrifugation?
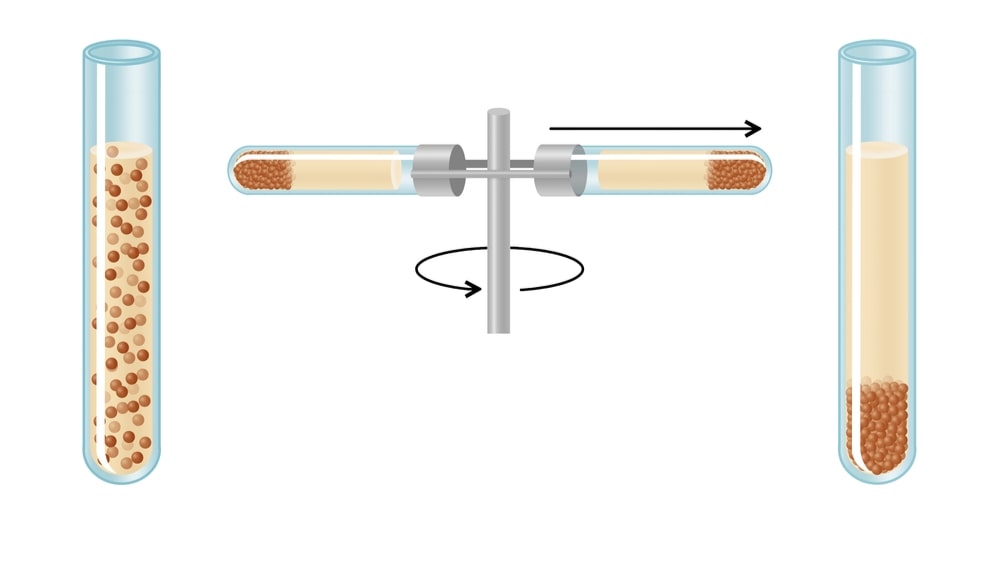
Centrifugation is a unit operation employed for separating the constituents present in a dispersion with the aid of centrifugal force.
Centrifugal force provides the driving force for the separation. It replaces gravitation forces operating during the sedimentation. Centrifugation is particularly useful when separation by ordinary filtration is difficult, for example, separating highly viscous mixtures and colloidal dispersions (particle size less than 5 mm), in which the difference in the densities is less. In short, centrifugation provides a convenient method of separating either two immiscible liquids or a solid from a liquid. The equipment used for the separation is known as centrifuges.
Process of Centrifugation
The centrifuge consists of essentially a container in which a mixture of solid and liquid or two liquids is placed and rotated at high speeds. The mixture is separated into its constituent parts by the action of centrifugal force on their densities. A solid or liquid of higher specific gravity is thrown outward with greater force. Therefore, it is retained at the bottom of the container leaving a clear supernatant layer of pure liquid.
The speed of a centrifuge is commonly expressed in terms of the number of revolutions per minute of the rotor.
Applications
Production of bulk drugs: The centrifugation technique is used to separate crystalline drugs such as aspirin from the mother liquor. Free-flowing product results due to the removal of traces of mother liquor and avoidance of effervescence.
Production of biological products: Most of the proteinaceous drugs and macromolecules are present as colloidal dispersion in water. By normal methods, it is difficult to produce them on a large scale. Centrifugal methods are used for the separation of these constituents from water. Insulin can be obtained in pure form by selectively precipitating other fractions of proteins and subsequently separating them by ultracentrifugation. Centrifugation is employed for separating the blood cells from the blood.
Biopharmaceutical analysis of drugs: Drugs present in the blood. tissue fluids and urine are normally present in the form of colloidal dispersions. Centrifugation is used for separating the drugs. This method is essential for the evaluation of pharmacokinetic parameters and bioequivalence studies.
Evaluation of suspensions and emulsions: The centrifugation method is used as a rapid empirical test parameter for the evaluation of suspensions and emulsions. Normally, creaming is a slow process in emulsions. This process can be hastened by inducing stress conditions (using a centrifuge). A stable emulsion should not show any signs of separation even after centrifuging at 2000-3000 revolutions per minute at room temperature.
Determination of molecular weight of colloids: Determination of molecular weight of a polymer is not possible by usual methods. Ultra centrifugation methods have used the determination of the molecular weight of serum albumin, insulin, and methylcellulose. Centrifugation (ultra-centrifugation) is also used for ascertaining the degree of homogeneity of the sample. For example, insulin is a monodisperse protein composed of two polypeptide chains, whereas gelatin is found to be a polydisperse protein with fractions of molecular weight 10,000 to 1,00,000.
Make sure you also check our other amazing Article on : Rotary Drum Filter