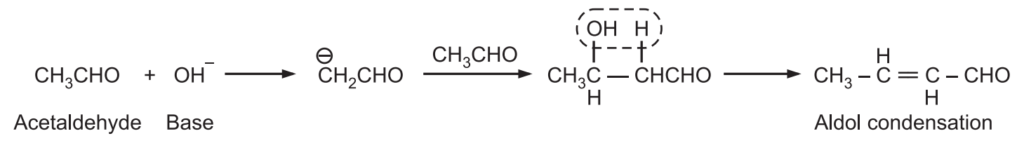
The condensation of an aromatic aldehyde with an aliphatic aldehyde or ketone in the presence of a base or an acid to form an α, β-unsaturated aldehyde or ketone is known as Claisen Schmidt Condensation. When an enolate is obtained from an aldehyde, normally reacts with unreacted aldehyde, the reaction is known as the Aldol condensation reaction.
When an enolate from a ketone reacts with an aldehyde, it is called Claisen-Schmidt condensation or crossed aldol condensation. In this, the product still has reactive alpha hydrogen and a hydroxide adjacent to it. Dehydration quickly occurs leading to the condensation product. The reaction is named after Rainer Ludwig Clasien in 1887.
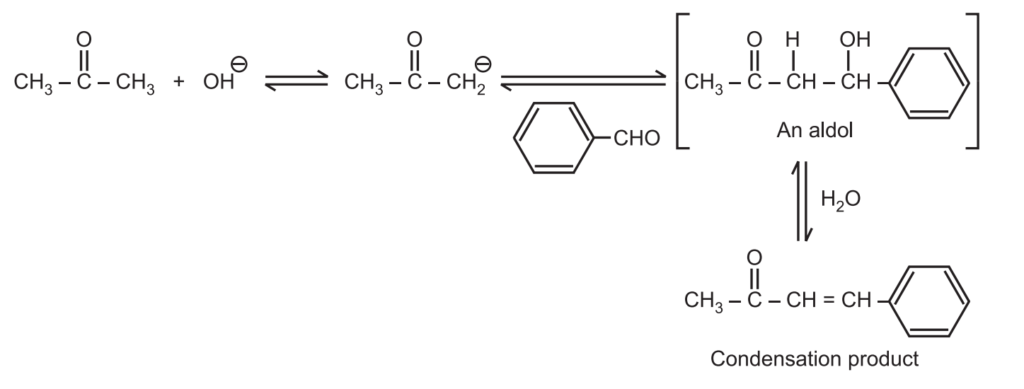
Sample reaction:
In the above-crossed aldol condensation:
(a) The Aldehyde carbonyl group is always more reactive towards nucleophilic addition than the ketone carbonyl group.
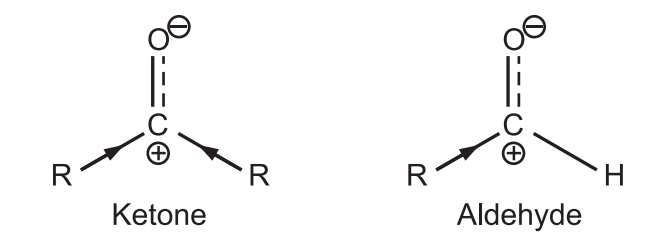
Alkyl groups are electron releasing in nature. The δ ⊕ (delta positive) charge developed on carbon of ketone is greatly neutralized due to more electron release by both alkyl groups. Hence, the carbonyl carbon of ketone is less reactive. While aldehyde has more reactive carbonyl carbon.
(b) Ketones form enolate ions more quickly than aldehydes.
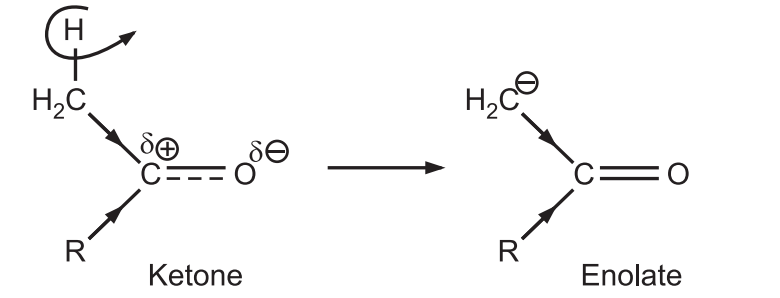
The ketone forms the lower energy enolate (nucleophile) at a much faster rate than aldehyde.
(c) Thus ketone generates enolates (nucleophile) while aldehyde has highly carbonyl carbon (electrophile). Hence in the “crossed/mixed” reaction, the ketone enolate usually adds to the aldehyde.
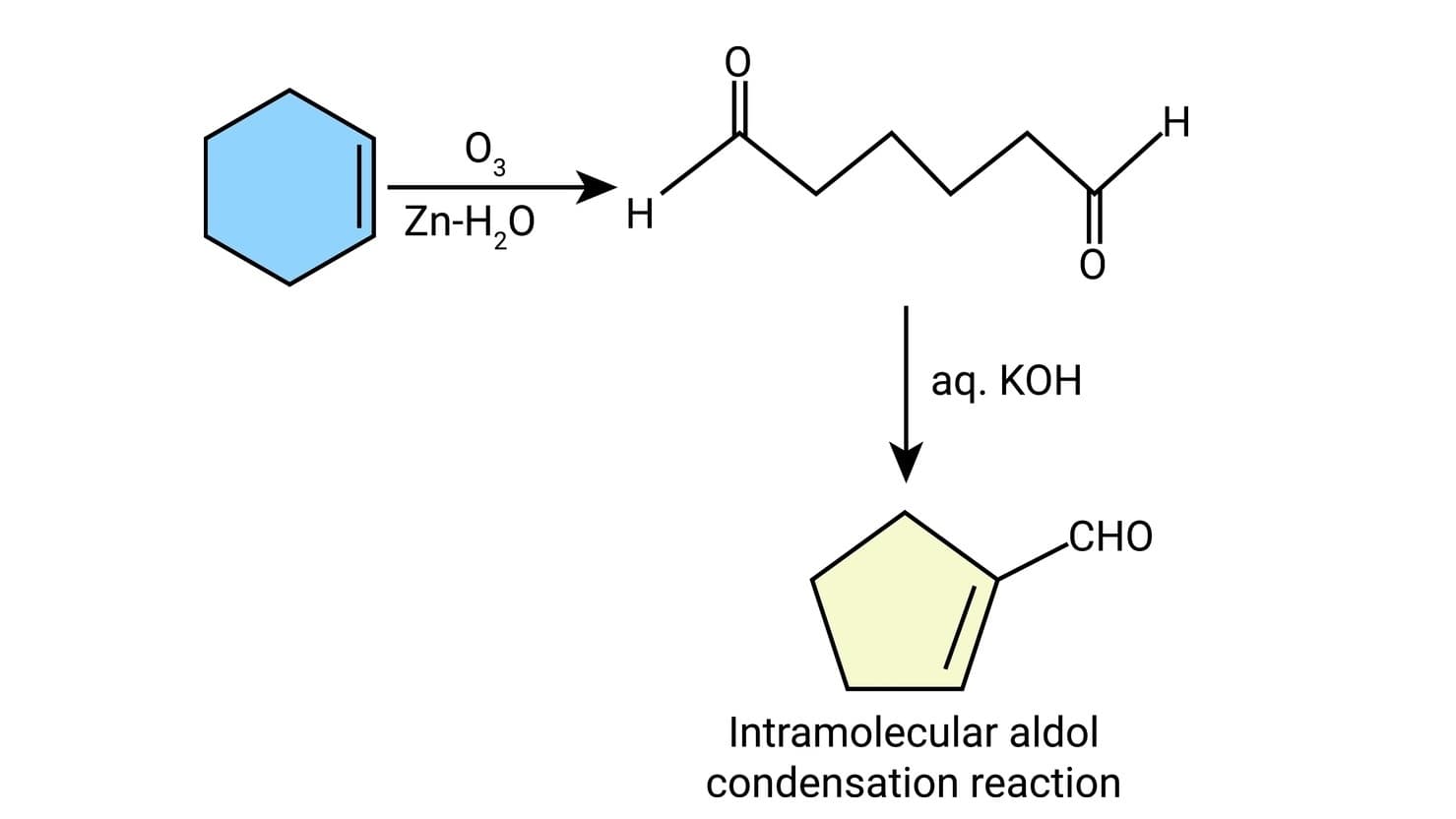
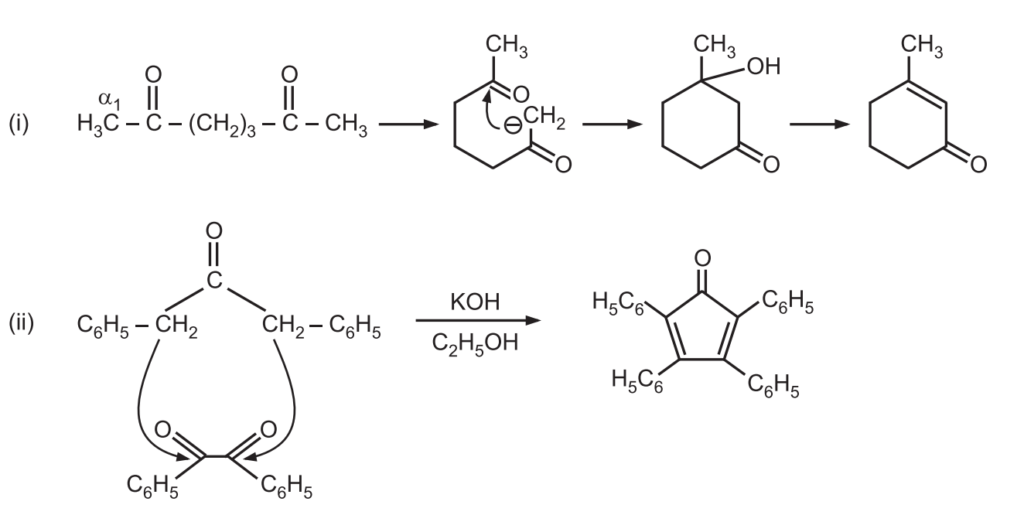
(d) When the reaction contains an enolizable aldehyde and a ketone, one product does predominate. This reaction may also be used in the formation of the ring structure. e.g.,
Example: Predict the major product of the following condensation
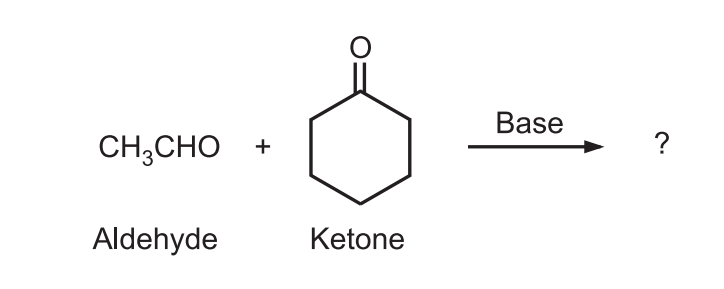
Ans.: Ketone generates enolate (nucleophile) that attacks highly reactive electrophile (carbonyl carbon) of aldehyde.
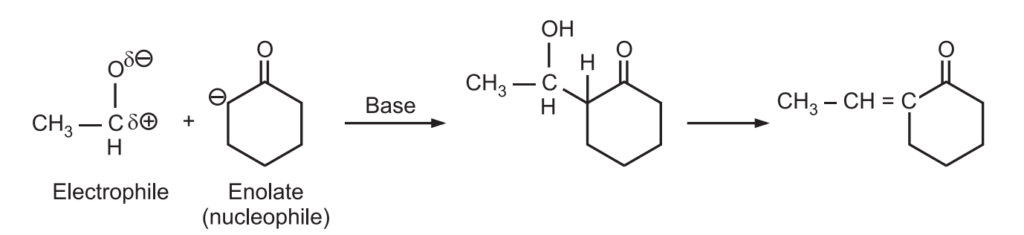
Mechanism of Claisen-Schmidt Condensation
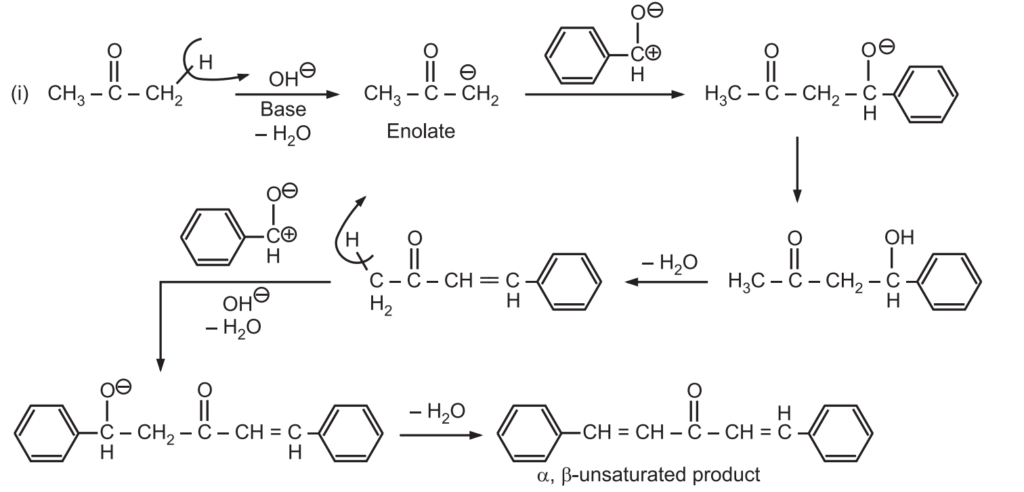
Applications in Drug Synthesis:
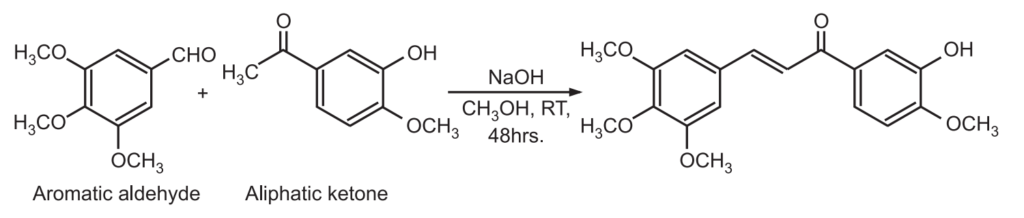
The Claisen – Schmidt condensation between acetophenone and benzaldehyde derivatives allows unsaturated ketone such as chalcones to be obtained.
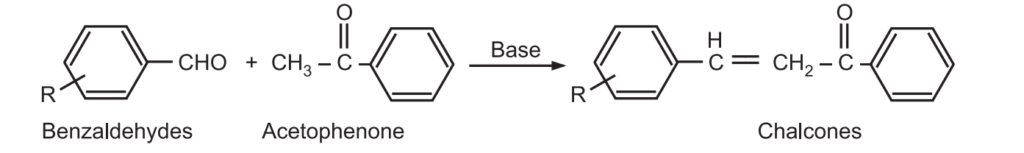
This reaction is also used to synthesize flavanone and 1.3-diaryl propane derivatives.
Make sure you also check our other amazing Article on : Schmidt Rearrangement