Table of Contents
What is meant by chromatography?
Chromatography is the separation technique used for the separation of the complex mixture. For example, in a tablet, the active ingredient is mixed with various excipients. In this, chromatography will be helpful to separate the excipient with other ingredients. Similarly, in-plant extract there are various phytoconstituents are present. The chromatography will be helpful to separate these phytoconstituents into their individuals.
Chromatography word was derived from the two words chroma and graphy. Chroma the greek word indicates color and graphy represent the meaning of writing and recording. The person who invented chromatography first was a Russian botanist Mikhail Tswett. He separated the chlorophyll and xanthophylls from the leaves of the plant by using a glass column packed with the fine particle of calcium carbonate. So the chromatography is a very primitive technique used for the separation of the mixtures.
Column Chromatography
What is Column Chromatography
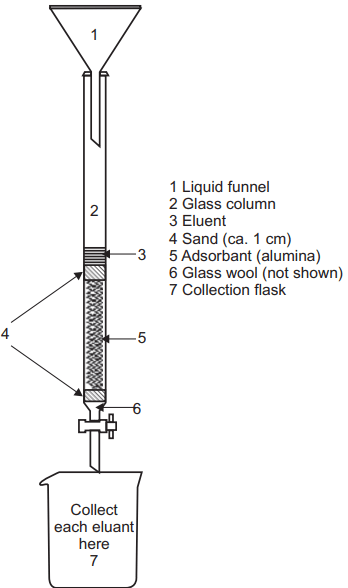
Generally in all the types of chromatography, there is one stationary phase and one mobile phase. In column chromatography, we use a packed column as a stationary phase. The column may be packed with solid or liquid. If the packing occurs with the solid the principle of separation is adsorption while if packing will be done by liquid then the principle of separation will be partition. Generally in column chromatography, the most separation is based on the adsorption principle. The adsorption principle is used in the separation of the mixture from column chromatography. The extract which is to be analyzed should be dissolved in the mobile phase and added into the column.
The individual constituent in the extract will move with different rates which depend upon their affinities towards the stationary phase or adsorbent. The compounds which have more affinity towards the stationary phase will be separated in the last while those that have less affinity towards the stationary phase will move fast and separated first.
The stationary phase which is used to fill the column should have a uniform spherical size with high mechanical stability, inert with solute or other components, and the mobile phase has a free flow, freely available, inexpensive, and able to separate the wide variety of compounds.
Example of weak adsorbent is sucrose, starch, inulin, talc while medium adsorbent is calcium carbonate, magnesium oxide, calcium hydroxide, and magnesium carbonate and the example of strong adsorbent is activated alumina, activated charcoal, fuller’s earth, etc.
The mobile phase may be used in individuals or can be used in the mixture depending upon their polarity and the type of constituent to be separated. The column should be made of neutral glass and not affected by acid, alkali, mobile phase, or stationary phase. The length: diameter ratio may be 10:1 to 30:1. Sometimes it may be a 100:1 ratio.
Packing of the Column:
The lower portion of the column should be packed with glass wool or cotton wool above which the adsorbent should be placed. After packing with the adsorbent, a paper disc can be placed above the adsorbent layer. So that the introduction of the sample or mobile phase will not disturb the adsorbent layer. There are generally two types of column packing techniques
- Dry packing technique.
- Wet packing technique.
In the dry packing technique, the column is packed with dry adsorbent and then flown the solvent through the column till equilibrium is obtained but the major drawback is that air bubbles are trapped between the solvent and adsorbent which produces trouble in the separation of compounds.
In the wet packing technique, the mobile phase is mixed with adsorbent and poured into the column. So the air entrapment is comparatively less and uniform packing occurs in the column. If the flow of the mobile phase will be uniform there will be no development of crack and good and uniform separation of the extract will occur.
Sample Introduction of Column Chromatography:
The sample should be dissolved in the least quantity of mobile phase. The complete sample should be placed on the top portion of the column and get them absorbed. Then the sample can be isolated by elution. Elution may be of two types:
- In Isocratic elution, the composition of the mobile phase will be the same for the entire separation.
- In gradient elution, the composition of the mobile phase can be changed slowly to improve the separation. The polarity of the mobile phase can be changed by changing the mobile phase combination.
Detection of Components:
Colored constituents can be detected visually and colored bands moving in the column can be collected separately. Colorless compounds can be detected by UV technique, refractive index detector, monitoring by TLC, by flame ionization detector, or such other technique. The component can be recovered by different techniques some time even by cutting the column (column made of plastic) into several distinct zones but the best method is elution in which the component is eluted by the mobile phase and the constituent is separated from the mobile phase.
Column Chromatography
Column Chromatography Applications
- Column Chromatography is used to isolate active ingredients.
- It is very useful helpful in separating compound mixtures.
- It can be used to determine drug estimation from drug formulations.
- It can also used to remove impurities.
- Used to isolate metabolites from biological fluids
Make sure you also check our other amazing Article on : Supercritical Fluid Extraction
