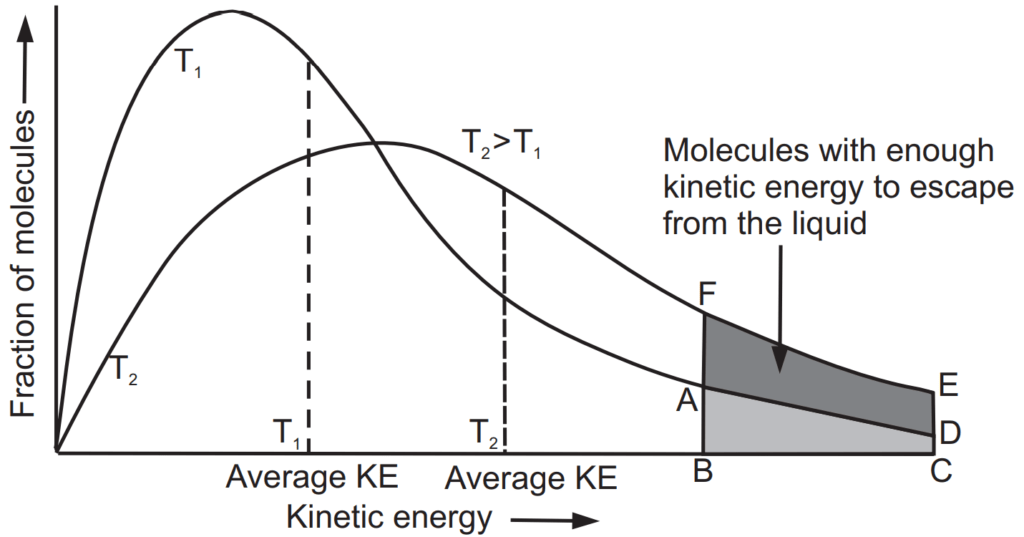
A liquid need not always have to be heated to its boiling point before it changes to a gas. The kinetic energy of the molecules is proportional to the absolute temperature of the gas. Due to high kinetic energy gas molecules are in a state of constant motion. In liquids, only a few molecules have lower or higher kinetic energy. It is illustrated in Fig. 1.1. At low temperature, the number of molecules having high kinetic energy is less as shown by ABCD while at high temperature the number of molecules having higher kinetic energy increases as shown by FBCE. The molecules with high kinetic energy are important to escape from the liquid state to the vapour state. Upon cooling, kinetic energy gradually decreases. Since the temperature has decreased a stage is attained at which gas molecules loses their energy that they are unable to overcome forces of attraction between them. This situation brings the gas molecules near to have contact with each other achieving a more condensed liquid state. This state also can be possible to achieve by the increasing pressure of the gas but it has a limitation that pressure is effective only below a specific temperature. This temperature is called as critical temperature. A critical point is defined as the temperature above which gas cannot be liquefied, even if very high pressure is applied.
The critical temperature of the water is 374 °C or 647 K and its critical pressure is 218 atm. If liquid such as water is sealed in an evacuated tube, a specific amount of it evaporates to produce vapour at a constant temperature. Like gas, water vapour exerts pressure and maintains equilibrium between liquid and vapour phases. Exerted vapour pressure is characteristic of every liquid and is constant at any given temperature.
The vapour pressure of water at 25 °C is 23.76 mmHg while at 10 °C it is 760 mmHg and therefore it is clear that vapour pressure increases continuously with temperature. As water is heated further, it evaporates to more amount resulting in increased vapour pressure.
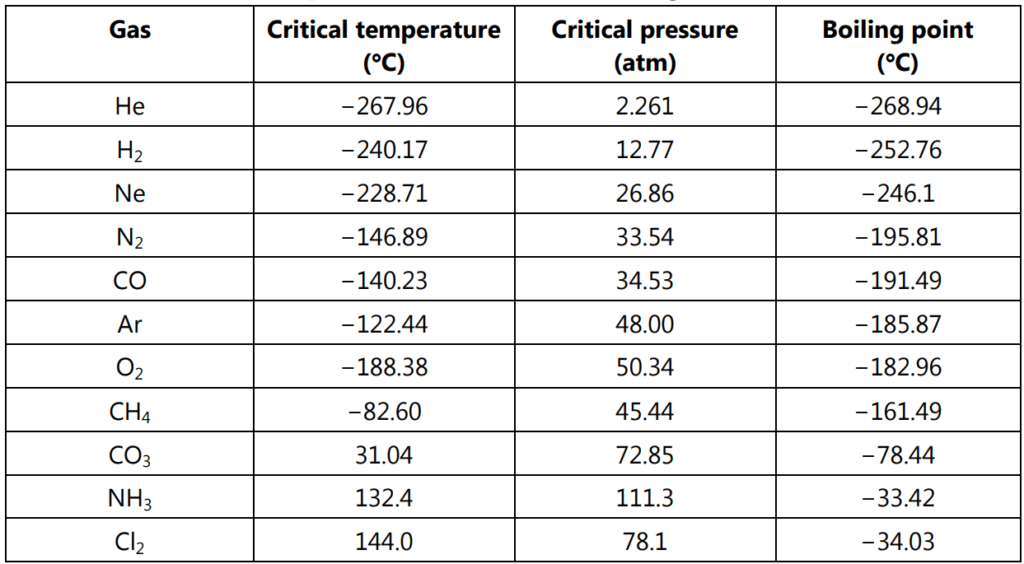
When the temperature reaches 374 °C the water meniscus becomes invisible. At the critical temperature, the physical properties of liquid and vapour become identical and no distinction can be made between the two. This point is also called as the critical point. The temperature, saturated vapour pressure and molar volume corresponding to this point are designated as critical temperature (Tc), critical pressure (Pc) and critical volume (Vc) respectively. For water these critical constants are; Tc = 374 K, Pc = 219.5 atm and Vc = 58.7 mL/mole. The critical points for different gases are given in Table 1.1.
Make sure you also check our other amazing Article on : Hard gelatin capsules