Aim: To determine the bulk density of magnesium carbonate.
Principle: Bulk density is the mass of a powder divided by the bulk volume.
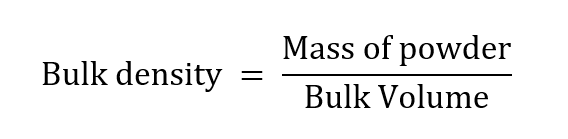
Apparatus and materials: Measuring cylinder, magnesium carbonate (MgCO3), and weighing balance.
Process:
- The powder whose bulk density is to be determined is passed through sieve no. 20.
- About 20 g is weighed accurately and carefully introduced into a 100 ml graduated measuring cylinder.
- The cylinder is dropped at 2-second intervals onto a hard surface three times from a height of I inch.
- The volume of powder is noted.
Observation and Calculation:
Weight of powder = W1 g
The volume of powder = V cc
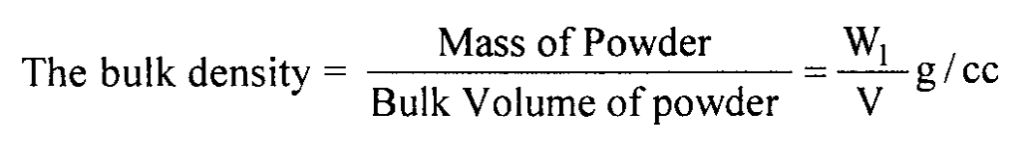
(Convert the value to SI units by multiplying by a factor of 103)
Report: The bulk density of magnesium carbonate is kg/m3.
Make sure you also check our other amazing Article on: Determination of Density