Aim: To determine the specific gravity of glycerin.
Principle: Specific gravity is the ratio of the density of a substance to the density of water. The density of both substances should be recorded at the same temperature unless specified. It is also called relative density. It is often defined as the ratio of the mass of a substance to the mass of an equal volume of water at 4 °C or at some specified temperature.
When volume is equal, specific gravity is the ratio of the weight of liquid to the weight of an equal volume of water.
Apparatus and materials required: Pycnometer (specific gravity bottle), glycerin, water, and weighing balance.
Process:
- The clean and dry empty specific bottle is weighed.
- Then the bottle is completely filled with distilled water and weighed.
- After cleaning and drying, the bottle is filled completely with the liquid whose specific gravity is to be determined (glycerin) and weighed.
Observation:
Room Temperature: ________________ °C
Weight of empty dry specific gravity bottle = W1 g
Weight of specific gravity bottle filled with water = W2 g
Weight of specific gravity bottle filled with glycerin = W3
Calculation:
Mass of water = W2 – W1 g
Mass of liquid = W3 – W1 g
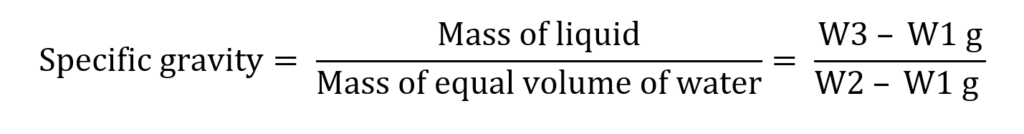
Report: The specific gravity of glycerin is ____ at ________ 0C.
(The specific gravity of glycerin is 1.16)
The specific gravity of a few liquids (Values Are Approximate).
| Ethyl alcohol | 0.79 |
| Acetic acid | 1.05 |
| Chloroform | 1.49 |
| Ethyl acetate | 0.90 |
| Castor oil | 0.97 |
| Olive oil | 0.92 |
Make sure you also check our other amazing Article on: Determination of Bulk Density
