Aim: To determine the constants of Freundlich adsorption isotherm for the adsorption of acetic acid on charcoal.
Principle: The phenomena of accumulation of solids, dissolved in liquids, at the surface of another solid are known as adsorption (more generally at the interface of two phases). The gases too adsorb to solid surfaces in a similar way. The adsorbate (that gets adsorbed) is adsorbed on the adsorbent (that adsorbs).
The extent of adsorption depends on the temperature. It decreases with the increase in temperature where adsorption is an exothermic process. At a given temperature, the amount of adsorption increases with an increase in the concentration of adsorbate (when dissolved in liquid) or pressure (when in the gas phase). Freundlich adsorption isotherm represents the variation in the extent of adsorption with the equilibrium pressure or concentration of adsorbate at a fixed temperature.
What is Freundlich constant?
The Freundlich adsorption isotherm for the adsorption of a solid from a solution is conveniently expressed as:
\log\left(\frac xm\right)=\log\left(k\right)+\;\frac1n\;\log\left(C\right)
Where,
x = Amount of adsorbate adsorbed by m g of adsorbent,
C = Equilibrium concentration of adsorbate,
k and n = Empirical constants.
The plot of log x/m (Y-axis) and log C (X-axis) makes a linear line with slope = 1/n and Y-intercept = log k.
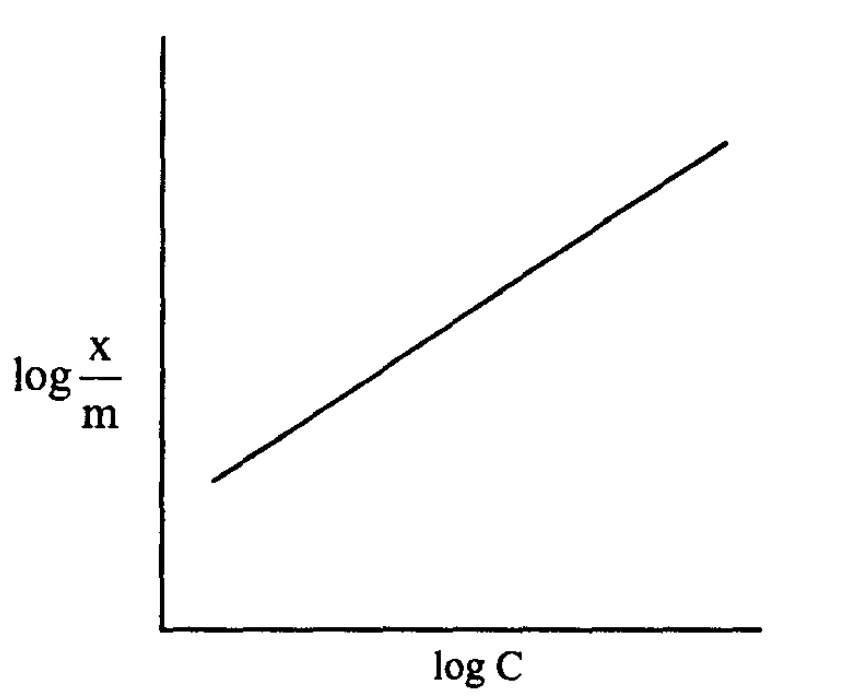
The value of k can be directly read from the Y intercept from a plot on log-log paper.
When charcoal is added to a solution of acetic acid, the acetic acid gets adsorbed on the charcoal. On filtration, the filtrate will contain acetic acid, which can be determined titrimetrically. If free acetic acid were subtracted from the known total amount of acetic acid, it would give the quantity of acetic acid adsorbed.
Apparatus and materials required: Activated charcoal, N/2 acetic acid, Nil ° sodium hydroxide, burette, pipettes, and reagent bottles.
Process:
- Around 2 g of powdered activated charcoal is accurately weighed and placed into each of the cleaned and dried bottles numbered 1 to 5.
- Using burette 50, 30, 20, 10, and 5 ml quantities of N/2 acetic acid are added into the bottle numbers 1, 2, 3, 4, and 5 respectively. Similarly, 0, 20, 30, 40, and 45 ml quantities of water are added to bottle numbers I to 5 respectively (to make volume equal in all bottles).
- The bottles containing charcoal, acetic acid, and water are vigorously shaken from time to time for about 30 minutes. The solution is then kept undisturbed for about 5 minutes.
- The solutions of each bottle are then filtered through dry filter papers and the filtrates are collected in properly labeled flasks, rejecting the first 5-10 ml of filtrate in each case.
- 10 ml of these filtrates are pipetted out into different conical flasks and titrated against N/10 sodium hydroxide using phenolphthalein as an indicator.
- 10 ml of stock N/2 acetic acid is also titrated separately using N/10 sodium hydroxide.
Observation and calculation:
Room temperature: ___ 00
10 ml of stock N/2 acetic acid solution = x ml of N/10 sodium hydroxide
| Flask | Amount of charcoal in g (m) | The initial concentration of acetic acid before adsorption | Equilibrium concentration after adsorption (Concentration in filtrate) (C) | Amount of acid adsorbed (x) | x/m | Log x/m | Log C |
| 1. | 0.5 M | ||||||
| 2. | 0.3 M | ||||||
| 3. | 0.2 M | ||||||
| 4. | 0.1 M | ||||||
| 5. | 0.05 M |
The concentration of acetic acid in filtrate can be determined from this relation:
1000 ml N sodium hydroxide \equiv\;1000 ml N acetic acid
(Normality and molarity value of acetic acid is the same)
Amount of acid adsorbed (x) =
\frac{(Initial\;concentration-Equlibrium\;concentration)\times Vol\;of\;acetic\;acid\;solution\;\times Mol.Wt\;}{1000}\;g
The volume in this experiment is 50 ml.
(Determination of slope is described separately in the appendix)
Slope = 1/n and the value of n can be calculated. And the k value can be determined from intercept log k.
Report: The value of n and k of acetic acid adsorption on charcoal adsorption isotherm is ______ and ____.
Make sure you also check our other amazing Article on: Determination of Viscosity