Aim: To prepare a flocculated and deflocculated suspension of magnesium carbonate and assess their stability
Principle: Magnesium carbonate is an insoluble but diffusible solid.
In flocculated suspension, the particles settle more quickly than particles of deflocculated one. But the sediments form a cake-like structure in deflocculated suspension making dispersibility on shaking a big problem. Comparing these two types of suspensions, the flocculated one is pharmaceutically acceptable or preferable. However, controlled flocculation is desirable to achieve controlled sedimentation with ease of dispersibility.
Electrolytes and ionic surfactants can be used as flocculating agents to produce a flocculated suspension. Suspension prepared without flocculating agents (drug and solvent) is a deflocculated one.
The physical stability of the suspension is assessed by determining the sedimentation volume and/or degree of flocculation.
The suspensions can be prepared by using the formulae:
| Flocculated Suspension | Deflocculated Suspension |
| Magnesium carbonate 5g. | Magnesium carbonate 5 g. |
| Aluminum chloride 0.1 g. | |
| Water to 100ml | Water to 100ml. |
Materials and apparatus required: Magnesium carbonate (MgCO3), aluminum chloride, water, pestle and mortar, and measuring cylinders.
Procedure:
Preparation of Suspension
(i) Step: Preparation of Suspension
Deflocculated Suspension
- The light magnesium carbonate is powdered in a mortar.
- The water is added with trituration to make a cream and diluted sufficiently.
- Then it is transferred to a measuring cylinder. The mortar is repeatedly rinsed with little water every time and the rinsed mixture is added subsequently to adjust the volume.
Flocculated Suspension
- The light magnesium carbonate is powdered in a mortar.
- Aluminum chloride is dissolved in little water and added with trituration to make a cream. The cream is transferred to the measuring cylinder after dilution with water.
- The mortar is repeatedly rinsed with little water every time and the rinsed mixture is added subsequently to adjust the volume.
(ii) Step: Evaluation of suspension
- The suspensions in the cylinders are thoroughly shaken to make the dispersions uniform.
- The cylinders are kept undisturbed on a flat surface after shaking.
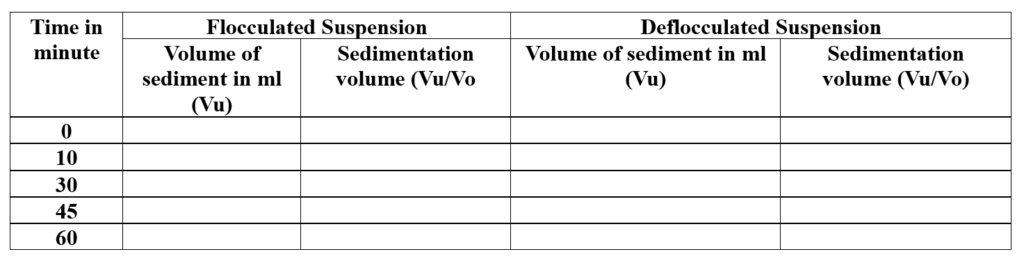
- The volume of sediment at different times: 0, 10, 30, 45, and 60 minutes is measured. (iv) The sedimentation volume and degree of flocculation are calculated.
Observation and Calculation:
The original volume of suspension = 100 ml.
The\;degree\;of\;flocculation\;at\;one\;hour=\frac{sediment\;volume\;of\;flocculated\;suspension\;at\;1\;hour}{sediment\;volume\;of\;deflocculated\;suspension\;at\;1\;hour}
(Though the degree of flocculation is related to the ultimate sediment volume in flocculated and deflocculated suspension, it is not feasible to achieve or determine the ultimate volume within the practical period. Hence the value at 1 hour is determined.)
The plot of sedimentation volume on the Y-axis and time on the X-axis is drawn.
Report: The flocculated and deflocculated suspensions are prepared and evaluated for sedimentation volume.
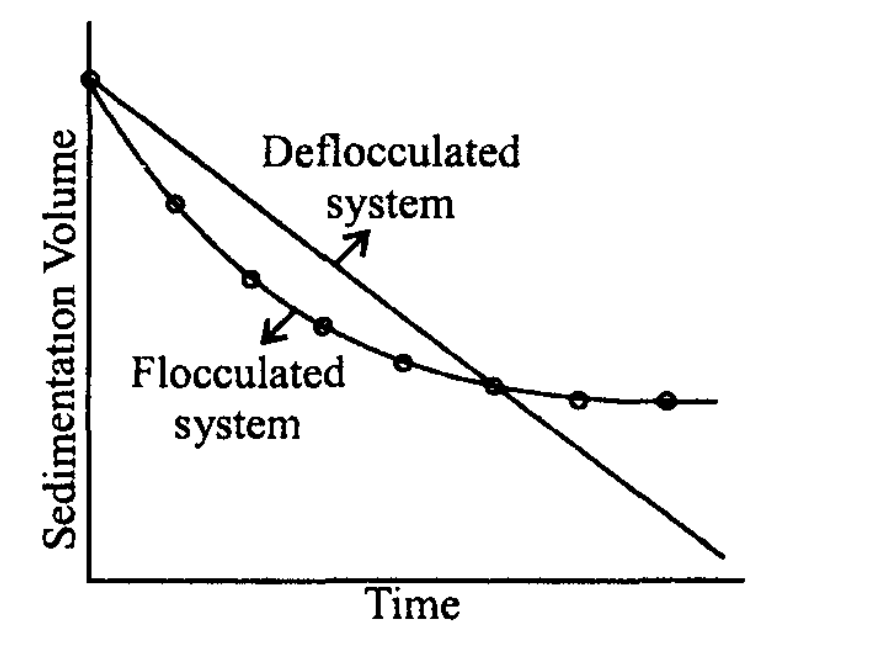
(Sedimentation volume quickly decreases in flocculated suspension compared to deflocculated suspension initially but the ultimate sedimentation volume of flocculated suspension will be higher).
Note: In formulation development, several suspensions with different formulas can be prepared and evaluated based on sedimentation volume to find out the best one. An assessment of the slope of each line gives an indication of which suspension shows the lowest rate of sedimentation. The more horizontal the curve is, the better the suspension.
Make sure you also check our other amazing Article on: Determination of Freundlich Adsorption Isotherm Constant
