Aim: To determine the surface tension of a liquid (glycerin) by drop counting method using a stalagmometer.
Principle of Drop Count Method
The ratio of the weight of a drop of the liquid (W1) to that of a reference substance (W2) falling from the same capillary orifice is equal to the ratio of their surface tensions.
If ϒ1 and ϒ2 are the surface tensions of liquid and the reference standard respectively. Then,
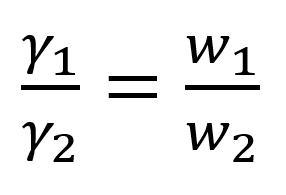
The weight of a single drop is equal to vp/n, where v is the volume of liquid delivered; n is the number of drops; p is density. Then the above equation becomes:
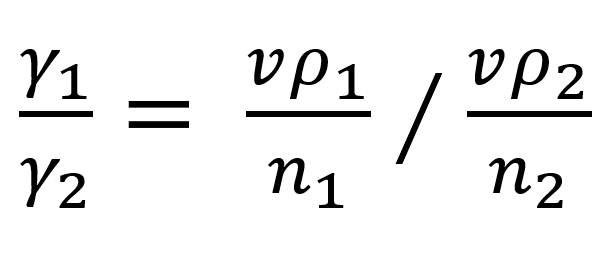
If the same volume of liquid and reference substance is allowed to flow from the same stalagmometer, then
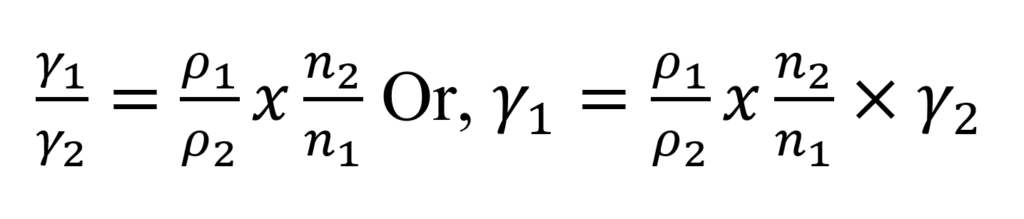
From the above equation, it is clear that if the densities and the numbers of drops of both liquids are known, the surface tension can be calculated.
Water is generally used as a reference liquid.
Apparatus and materials required: Stalagmometer, glycerin, water, specific gravity bottle, and balance.
Process:
- The cleaned stalagmometer is fixed perpendicularly with a clamp & stand and then filled with water by sucking.
- The flow rate of water is controlled at the rate of 10-15 drops per minute by keeping a clamped rubber tube or fingertip at the top.
- The number of drops is counted as they fall when the meniscus passes between mark C and 0 (fixed volume). The process is repeated to collect at least three readings.
- The stalagmometer is then dried and the experiment is repeated with the liquid whose ST is to be determined.
Observation and Calculation:
Room temperature: ___ 0C
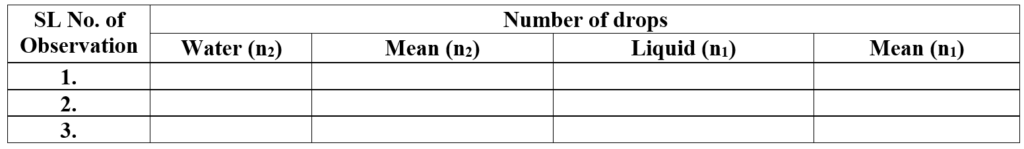
Other data required are the Density of the liquid and water (the reference standard), which can be determined following the procedure, described under density determination experiments.
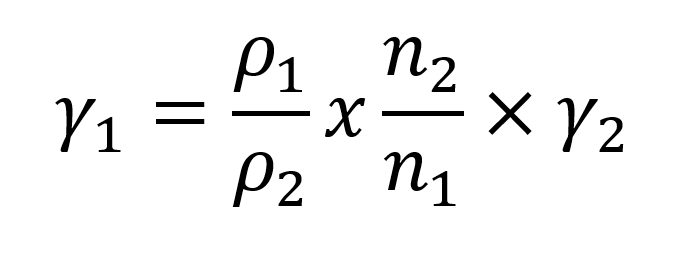
Report: The surface tension of the liquid (glycerin) is ___ mN/m at _________0C
Make sure you also check our other amazing Article on: Determination of Surface Tension by Drop Weight Method
