Aim: To determine the surface tension of a liquid (glycerin) using a stalagmometer by drop weight method.
Principle of Surface tension
The Surface tension (ST) measurement involves counting the number of drops formed when a definite volume of liquid is allowed to flow slowly out of a capillary orifice (stalagmometer). The ratio of weight (wI) of a drop of the liquid to that of a reference substance (w2 ) falling from the same capillary orifice is equal to the ratio of their surface tension.
The weight of the drop in mg of a test liquid, W1=2πrϒ1
Correction factor: The correction factor depends on the radius of the tip, and the cube root of the volume of the drop.
The weight of a drop of the reference liquid, W2=2πrϒ2 correction factor (from the same capillary).
As the same apparatus is used for both liquids, the correction factor is the same assuming that the drop volumes are not different.
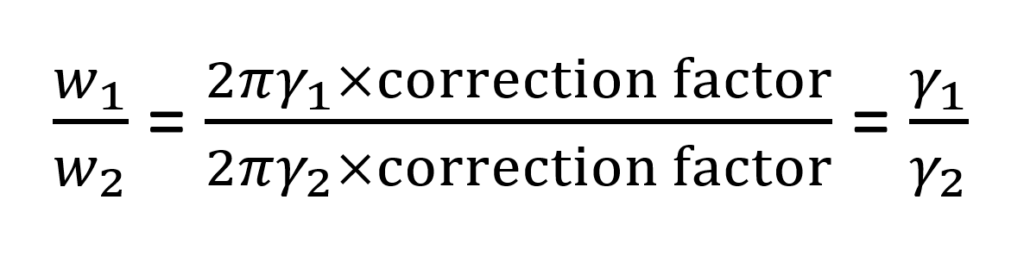
ϒ1 and ϒ2 are the surface tension of the test liquid and reference liquid respectively.
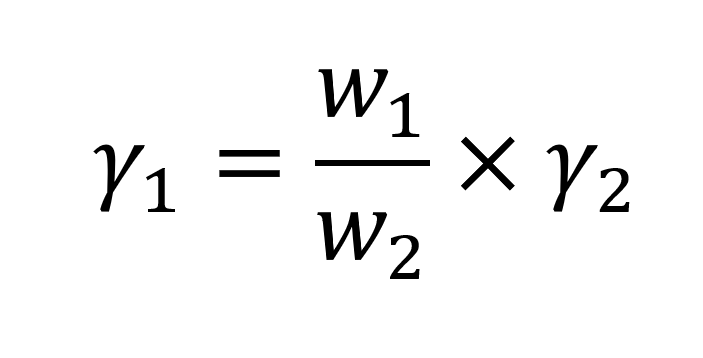
The value of the ST of the liquid can be calculated by taking two observations: the weight of one drop of liquid and that of a reference liquid if the ST of the reference substance is known. Water is usually used as a reference liquid.
Apparatus and materials required: Stalagmometer, glycerin, water, balance, weighing bottle.
Process:
- The cleaned stalagmometer is fixed with a clamp and filled with water by sucking.
- Around 20-30 drops of water, falling from the stalagmometer are collected in a dry and tared (preweighed) weighing bottle, and weighed.
- The stalagmometer is then dried and the experiment is repeated with the liquid whose ST is to be determined by a stalagmometer by drop weight method.
Care should be taken during an experiment that the number of drops does not exceed 20 per minute.
Observation and Calculation:
Room temperature: ____ °C.
- Weight of the clean and dry weighing bottle = X1 g.
- Weight of the dry weighing bottle + 20 drops liquid = Y1 g.
- Weight of the clean and dry weighing bottle = X1 g.
- Weight of the dry weighing bottle + 20 drops of water = Y2 g.
Therefore, weight of 20 drops of liquid = (Y1– X1) g
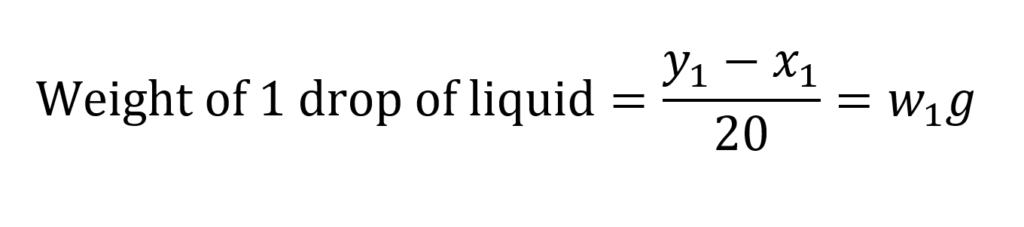
Similarly,

Then,
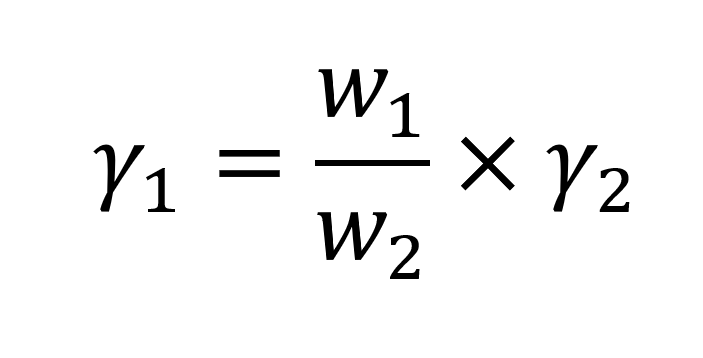
ϒ2 where ϒ2 is the surface tension of water which is taken as 72mN/m.
Report: The surface tension of the liquid (glycerin) is ____ mN/m at____________ °C.
Notes: ST of few common liquids at 20°C in mN/m
| Water | 72 |
| Glycerin | 63.4 |
| Liquid paraffin | 33.1 |
| Benzene | 35.0 |
Make sure you also check our other amazing Article on: Determination of Hydrophilic-Lipophilic Balance Value
