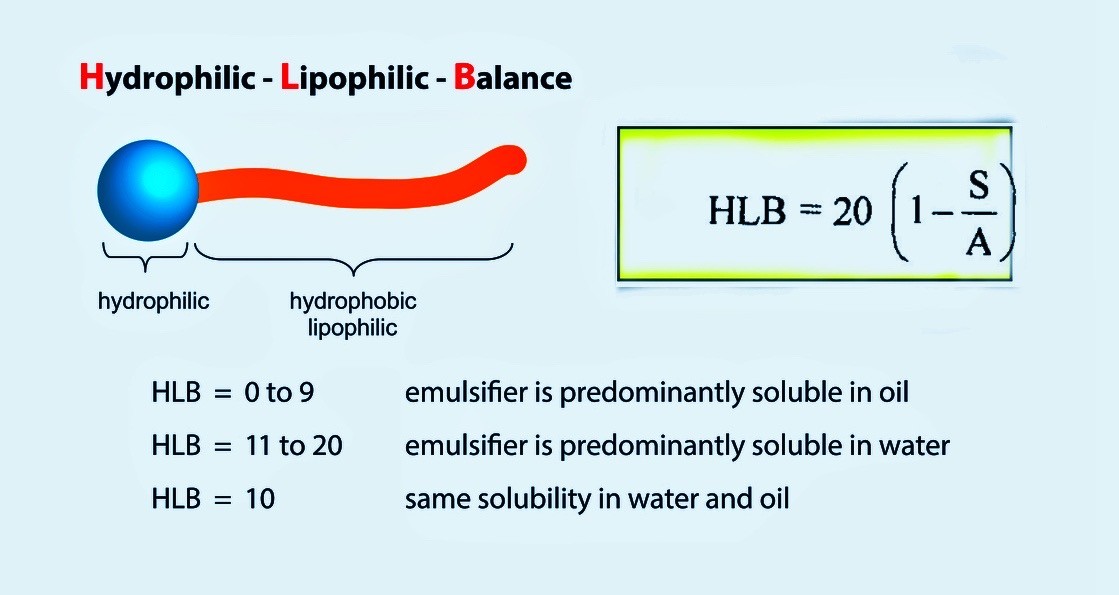
Aim: To determine the Hydrophilic Lipophilic Balance Value (HLB number) of polyoxyethylene sorbitan monolaurate (Tween 20).
Principle: The hydrophilic-lipophilic balance (HLB) of a surfactant is a measure of its polarity. The HLB value of non-ionic surfactant can be calculated using the formula:
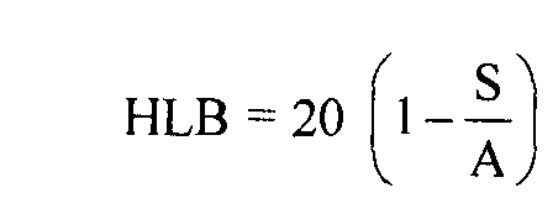
where S is the saponification number of esters and A is the acid number of fatty acids.
Apparatus and Materials required: Round bottom reflux, condenser, alcoholic potassium hydroxide, phenolphthalein, lauric acid, standard N/2 hydrochloric acid, and standard NIIO potassium hydroxide.
Process: It is restricted to the determination of saponification number and acid number.
A. Determination of saponification number:
- Around 1 gram of sample (Tween 20) is accurately weighed and transferred to a round bottom flask. 30 ml alcoholic potassium hydroxide (2.82%) is added and refluxed in a boiling water bath for about 1 hour.
- A blank experiment is performed in the same way but without using the sample.
- The reaction mixtures are cooled down to room temperature and titrated against standard N/2 hydrochloric acid using phenolphthalein as an indicator taking color change from pink to colorless or slightly yellow as the endpoint.
B. Determination of acid number:
- Around 500 mg of lauric acid is accurately weighed and mixed with 10 ml alcohol and 10 ml ether in a conical flask (slight warming may be necessary to dissolve lauric acid).
- The above mixture is titrated against standard N/1 0 potassium hydroxide using phenolphthalein as an indicator.
Observation and Calculation:
Weight of sample taken for saponification number determination = _________g.
Weight of sample taken for acid number determination = ____ g.
Determination of saponification number:
Let titre value of sample = V1 ml.
Titre value of blank = V2 ml.
(V1 – V1) ml of N/2 hydrochloric acid equivalent potassium hydroxide is required to neutralize the taken sample.
(V1 – V1) ml of N/2 hydrochloric acid = (V1 – V1) ml of N/2 Potassium hydroxide
1000 ml N Potassium hydroxide = 56000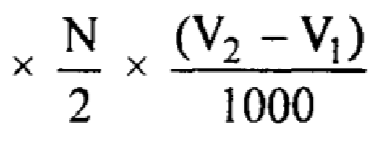 mg of Potassium hydroxide
mg of Potassium hydroxide
Note: The saponification number is defined as the number of mg of potassium hydroxide required to neutralize the acid present in one gram of substance.
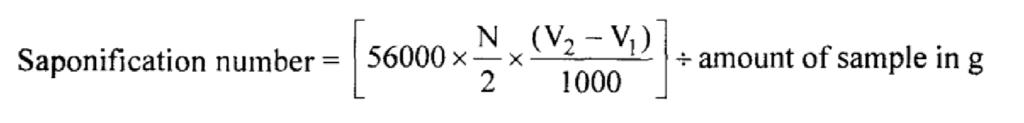
The acid number is defined as the number of mg of potassium hydroxide required to neutralize the free acid present in one gram of substance.
This formula can be written in a simplified way:
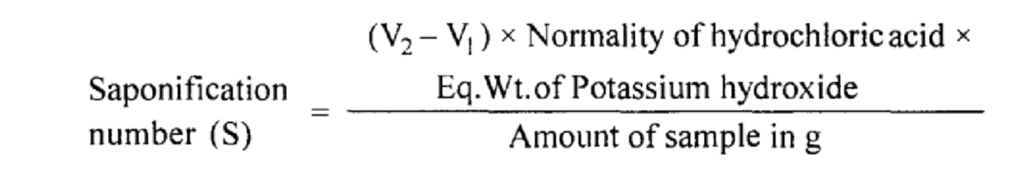
Determination of acid number:
Let the titre value of N/10 potassium hydroxide = V3 ml.
V3 ml of N/1 0 potassium hydroxide is required to neutralize the free acid in the taken sample.
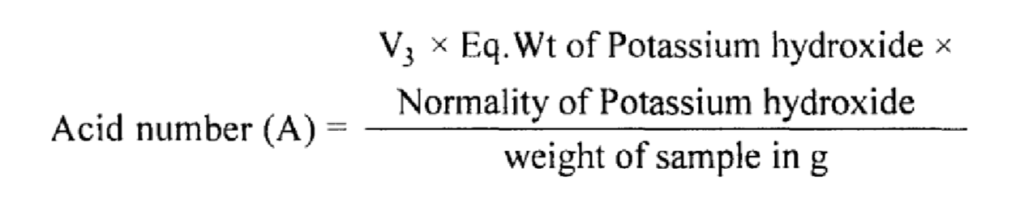
HLB = 20(1-S/A)
Report: The HLB number of polyoxyethylene sorbitan monolaurate (tween 20) is__________. (The normal values: S = 45.5, A= 276 and HLB = 16.7)
Make sure you also check our other amazing Article on: Determination of Dissociation Constant