Aim: To determine the dissociation constant (pKa) of a weak acid (salicylic acid).
Principle of Dissociation Constant
Principle: Weak acids and weak bases do not ionize completely in aqueous solutions like strong acids and bases. The degree of ionization is expressed in terms of ionization and dissociation constant, which is expressed conveniently in terms of pKa for both acids and bases. pKa is the negative logarithm of acid dissociation constant Ka in the same way as pH is used to represent the negative logarithm of hydronium ion concentration.
The dissociation constant value (pKa) for weak acids or bases can be determined using Henderson – Hasselbalch equation:
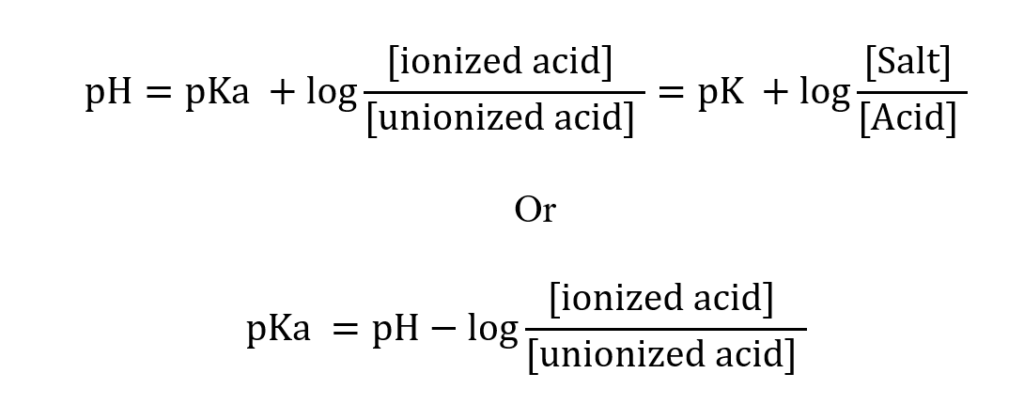
At the 50% neutralization point, that is when [ionized acid] = [unionized acid], the value of pKa = pH
The value of pKa can be directly measured by recording the pH at a 50% neutralization point.
Materials and apparatus required: Salicylic acid, standard sodium hydroxide solution, pH meter, conical flask, burette, pipettes, and indicator.
Procedure:
- 10 ml of O.5%w/v solution of salicylic acid in methanol is pi petted out to a conical flask. (Should not be sucked by mouth as methanol is toxic; a bulb pipette should be used).
- This is then titrated against O.5 N sodium hydroxide solution using methyl red as an indicator to complete neutralization. The burette reading (consumption of sodium hydroxide) is noted.
- Similarly 10 ml of O.5%w/v salicylic acid solution is taken into a flask and titrated against standard O.5N sodium hydroxide solution to 50% neutralization point (that is, half of the sodium hydroxide consumed in the previous titration is added).
- The pH of this half-neutralized solution is recorded using a pH meter.
Observation and Calculation:
Room Temperature: ____ 0C.
Let, the volume of O.5 N sodium hydroxide consumed in titration with 10 ml O.5% salicylic acid solution (complete neutralization) = Let’s say x ml
The volume of O.5N sodium hydroxide solution required for half neutralization = x/2 ml.
The pH of half neutralization solution = _________
Report: The pKa value of salicylic acid at 0C is _______(the normal value is 2.97)
The pKa value of some weak acids and bases:
| Weak acids | Weak bases |
| Paracetamol: 9.92 Aspirin: 3.49 Benzoic acid: 4.2 | Glycine: 2.35 Atropine: 9.68 Caffeine: 3.6 and 0.6 Codeine: 0.82 Urea: 0.18 |
Make sure you also check our other amazing Article on: Determination of Flow Properties of Powders by Carr’s Index