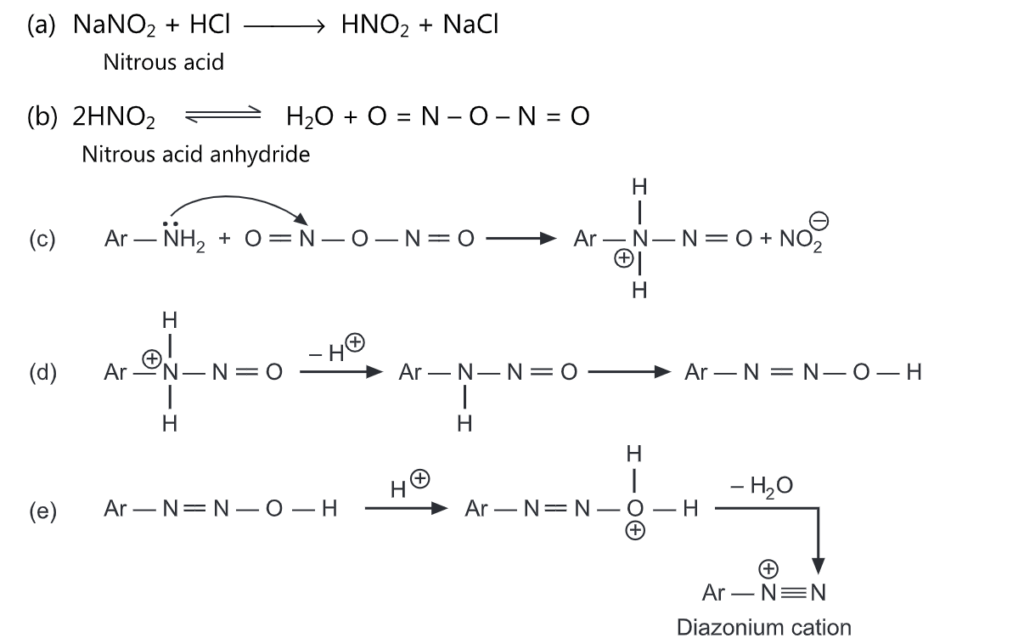
Diazo Coupling Reaction: The diazotization reaction, discovered by Griess in 1858, consists of the formation of diazonium salts when primary aromatic amines interact with nitrous acid in an acidic medium.
Diazo coupling comprises of diazotization of primary amine and coupling of diazotized salt with phenols. Since diazonium cation is a weak electrophile, it can only attack highly activated aromatic rings like phenols or amines resulting in the formation of the azo compounds Ar – N = N – Ar.
The diazo coupling reaction occurs easily with phenols and with somewhat greater difficulty with amines. The azo compounds are very stable and are usually brightly colored. Their color is due to the presence of a chromophore (-N = N-) in the molecule. The diazo coupling reactions are routinely used in the manufacture of azo dyes. Primary amines react with sodium nitrite and dilute HCI to form a diazonium cation. The reaction is carried out at a low temperature usually around 0°C, because of the relative instability of these salts.
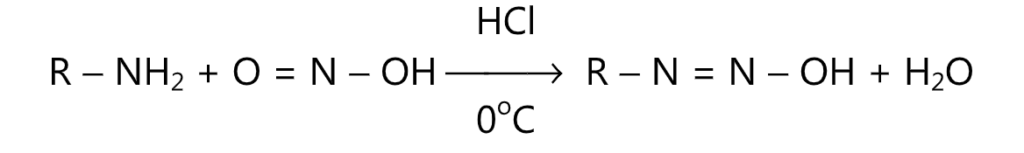
The aliphatic diazonium cations are less stable than aromatic diazonium cations where a stabilizing effect is provided by the л-orbital system of the nucleus.
The general mechanism of diazotization includes:
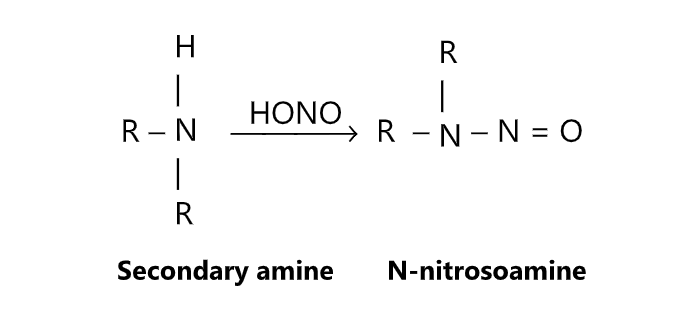
Secondary amines react with nitrous acid to give N-nitroso amines while tertiary amines do not react with nitrous acid. Nitrosation of the ring may often result if an aromatic tertiary amine is used.
In a strongly acidic medium, neither aromatic amine nor phenol is capable of undergoing a coupling reaction due to the very low concentrations of free amine (owing to the formation of diazonium salts) and the phenolate ion (phenol dissociation is strongly suppressed). In a strongly alkaline medium, a free base known as diazohydroxide (Ar – N = N-OH) separates from a diazonium salt which then forms a salt (Ar – N = N – ONa) that is not capable to undergo azo coupling.
Since diazonium cations exist in acidic or slightly basic solutions, the coupling reactions are carried out under these pH conditions. For example, coupling of the diazotized cation with phenoxide ion takes place in a basic medium.
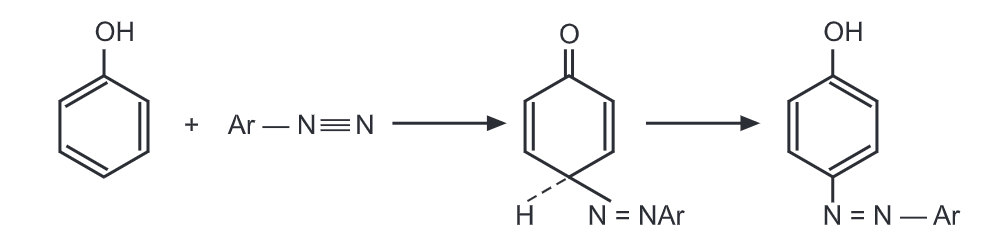
Because of the bulkiness of attacking electrophile, ArN2+, coupling normally takes place at the para position. Primary and secondary arylamines in slightly acidic conditions undergo coupling with diazonium cation.
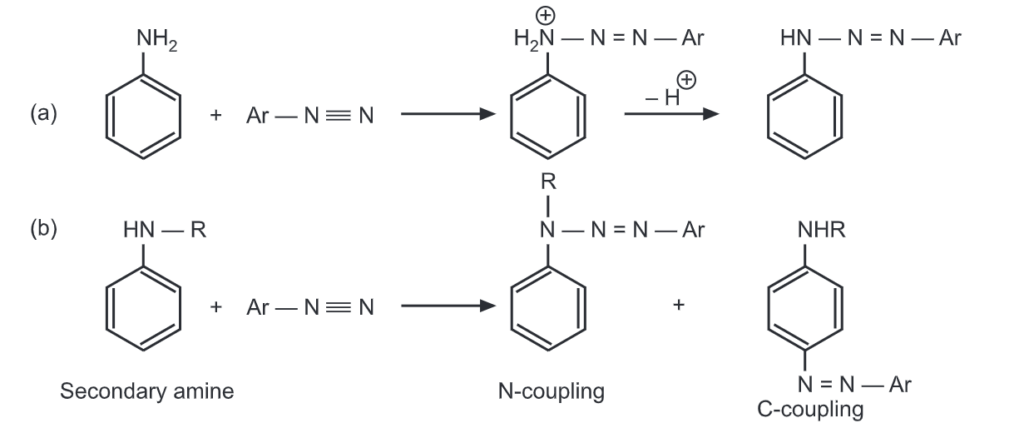
The primary amines undergo a coupling reaction at nitrogen. With secondary amine, some coupling may also occur at the carbon atom of the ring. With tertiary amine, coupling takes place only on the carbon atom of the ring.
Amines that form diazonium salts are called diazo components of a dye, while phenols and amines which enter azo coupling are called azo components. The activity of diazo components is enhanced by halogens and electron-withdrawing groups. These substituents reduce the activity of azo components.
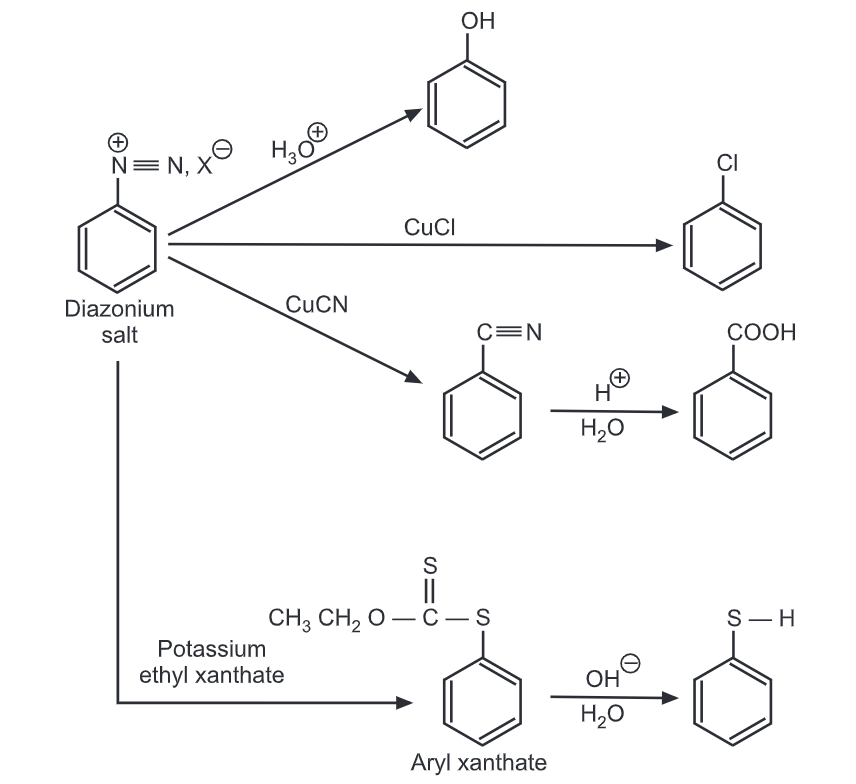
Upon warming in an acidic solution, the diazonium compound may be transferred to phenols, aromatic amines or other suitable functional groups added to the solution. Diazonium salts from primary aromatic amines are versatile synthetic tools to get a wide variety of organic compounds.
Make sure you also check our other amazing Article on : Nitrosation