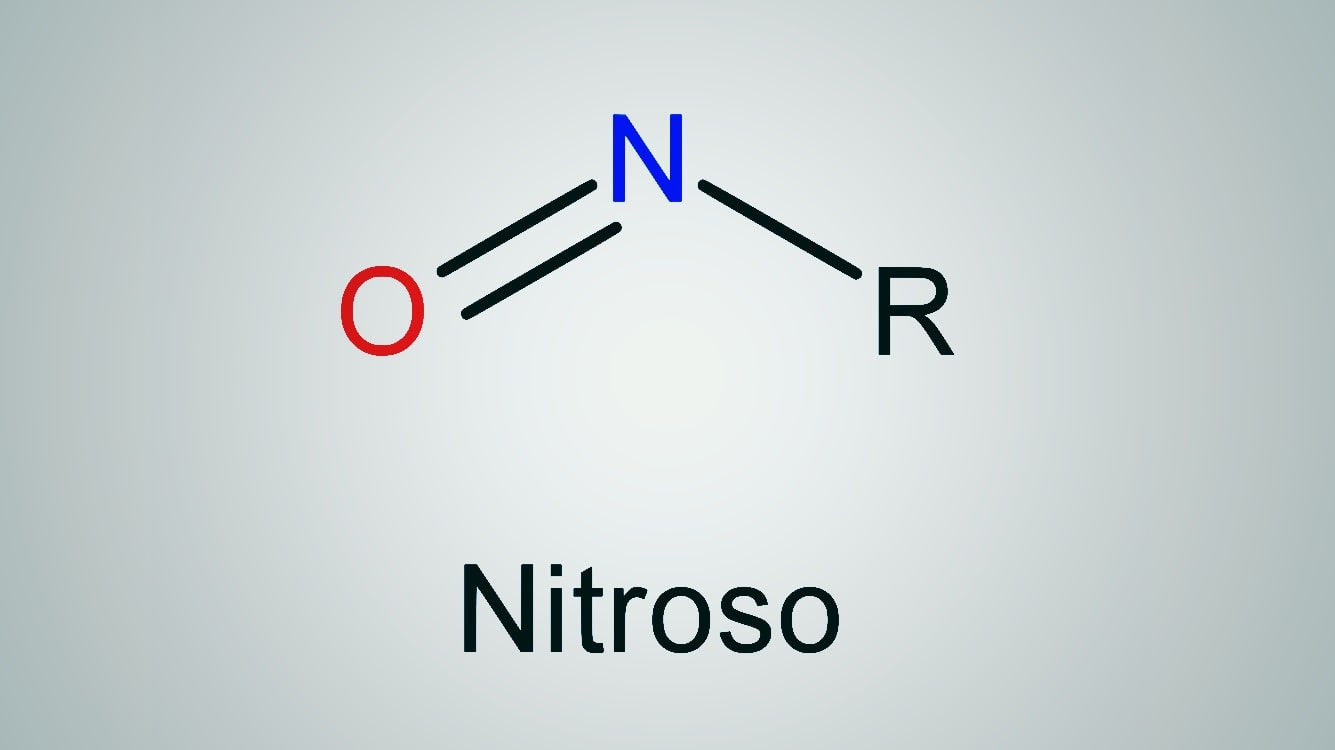
Nitrosation is an electrophilic aromatic substitution reaction in which the nitrosonium ion, NO is an effective electrophile. The nitrous acid (HNO3) is generated in both diazotization and nitrosation reactions. It is converted to nitrosonium ion through the following steps.
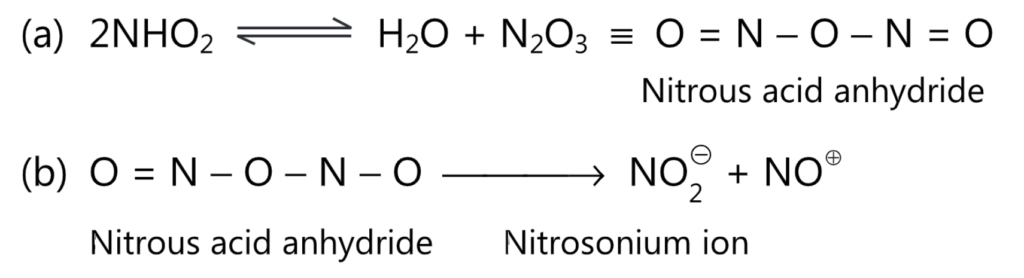
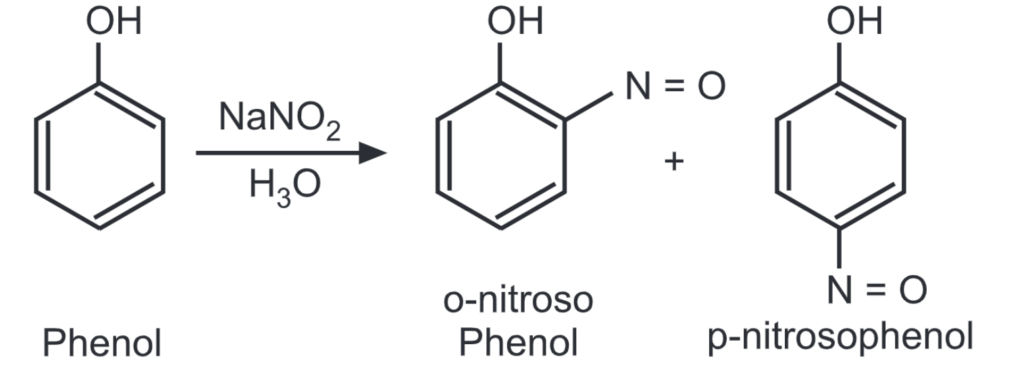
In fact, in many diazotization reactions of primary amines, nitrosonium ion may be acting as a reactive species. For nitrosation to occur on an aromatic ring, the ring should be activated by the presence of strong electron-releasing substituents (i.e., phenols).
Nitrosonium hexafluorophosphate NO+ PF6– may also be used as an effective nitrosating agent.
Make sure you also check our other amazing Article on : Friedel Crafts Acylation