Disinfectants are the chemical agents that are used to kill microorganisms (except spores, viruses, and prions) on inanimate objects (the things that are not alive) such as instruments and surfaces by means of physical or chemical processes. But they are not to be used on living tissues such as skin or mucous membranes.
The process by which destruction or removal and killing of all pathogenic organisms are carried out is known as disinfection.
The main purpose is to prevent the transmission of certain microorganisms with objects, hands, or skin and prevent the spreading of infection.
Table of Contents
Ideal Properties of Disinfectants
- They should have a wide spectrum of activity, should be active against all pathogens.
- They should be cidal (capable of killing bacteria) and chemically stable.
- They should be cheap and should not produce any stains.
- They should not damage any non-living materials.
- They should act in presence of organic matters.
- They should have high penetrating power and rapid action.
- They should be non-toxic and non-corrosive.
- They should be relatively safe for humans and other animals.
- They should be easily soluble in hard water and should be active at any pH.
- They should have good cleaning properties and should be non-inflammable.
- They should not damage the environment on disposal.
Classification of the Disinfectants
Disinfectants are broadly classified into two categories viz. physical methods and chemical methods. The overall classification is listed in Flowchart 1.
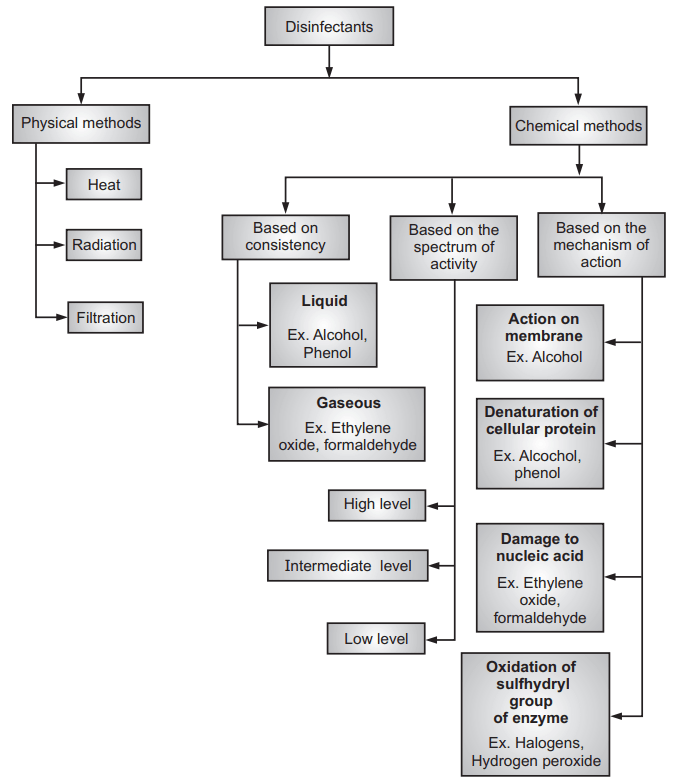
Physical Method
Heat:
Mainly dry and moist heats are used for the disinfection action. Flaming is used for the dry heat process whereas temperatures at various levels are used in moist heat sterilization. In moist heat sterilization, pasteurization is mainly used for disinfection where a temperature below 100°C is applied. This pasteurization is a process that is intended to reduce spoilage organisms and eliminate vegetative bacteria but not bacterial spores in certain foods and beverages. Pasteurization of milk was used first at temperatures of about 63°C for 30 minutes or heating to a higher temperature, 72°C, and holding for 15 seconds for disinfection.
Filtration:
This method is carried out for fluids that are heat-labile like antibiotics, vitamins, and other growth factors with very fine pore membrane filters below 0.45 microns in diameter. These fluids are checked for sterility by subculture before use. Filtration of air is necessary for the disinfection of the laboratory. Sterile filters are used for the separation of toxins and other soluble products of bacterial growth and are also used for the purification of water. Sometimes syringe filters are used for disinfection of a small volume of fluids. Membrane filters (pore size of 0.015 to 12 µM) are used for the preparation of parenteral and remove bacteria and yeast from the solution. The HEPA filter is used for the disinfection of air. The HEPA filtration reduces the number of organisms on dust particles by 99.9% down to 0.3-micron size.
Radiation:
Ultra-violet lights are used in laboratories that reduce the number of organisms to a low level in the air and on surfaces.
Chemical Methods
Based on Consistency:
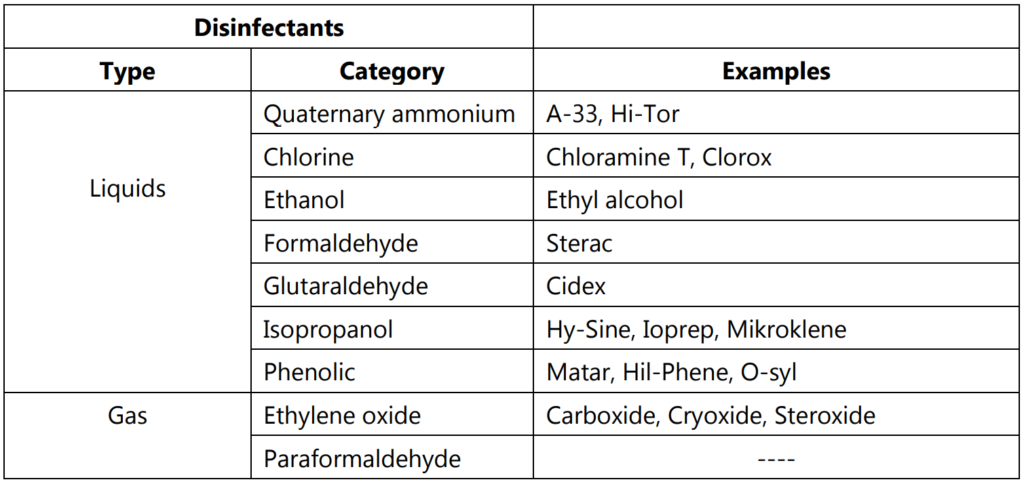
Mainly liquids and gaseous substances are used for the disinfection activity. Liquids like alcohol and phenol are widely used as a disinfectant. Alcohols like ethanol or isopropanol at a concentration of 50-70% are used for disinfectants. They are used for the disinfection of the surfaces and other laboratory equipment. Alcohols are low in sporicidal activity.
The phenolic chemicals contain a benzene ring with hydroxyl (OH) group which are more common disinfectants for environmental surfaces. Cresol is more powerful than phenol and a more commonly used compound which is diluted out further (1: 128 – 1 : 256) to achieve its bactericidal activity.
Gaseous agents like Ethylene oxide and aldehydes are used as disinfectant agents. Ethylene oxide is used as a sterilant, but in liquids, it shows antimicrobial action. Formaldehyde and glutaraldehyde are used as powerful disinfectants. The glutaraldehyde is the basic chemical for various marketed compounds like Cidex, or Sonacide, Sporocidin, or Glutacide. These are used for disinfectants of instruments, pipettes and clinical thermometers, etc.
Based on the Spectrum of Activity:
Based on the activity, disinfectants are classified as high, intermediate, and low levels. High level disinfectants such as aldehydes and gases, are used for endoscopes and disinfectants for surgical instruments. Some examples of high level disinfectants are like 2% glutaraldehyde for 20 minutes, 6% hydrogen peroxide for 30 minutes, 0.2% peracetic acid for 30-45 minutes. Intermediate level disinfectants are like alcohols and iodophors, used for disinfectant of the laryngoscope. Thereafter low level disinfectants are like quaternary ammonium compounds. They are also known as quats. They irreversibly bind to the phospholipids and proteins of the membrane. Earlier experiments utilized benzalkonium or cetylpyridium chlorides which act as good disinfectants but in low concentrated solutions, they become contaminated. Quaternary ammonium compounds are used as disinfectants of electrocardiograms and stethoscopes. Some examples of low level disinfectants are like 3% hydrogen peroxide solution for 10 minutes, 1000 ppm hypochlorite solution, 60-95% alcohol for 10 minutes.
Based on Mechanism of Action:
- Action on the membrane: Mainly alcohols such as ethanol, isopropanol, and methanol are used for this purpose. They act on the membrane of microorganisms and destroy the cell membrane. They are highly active in combination with water. 70% solution of Ethanol and isopropanol are used as hand disinfectants in pharmaceuticals. Example: Triclogel contains 75% ethanol.
- Denaturation of cellular proteins: Alcohols also denature the cell wall proteins. Phenols such as chlorocresol and chloroxylenol are used as disinfectants. They also denature the proteins and enzymes of the cell. Examples: Dettol contains chloroxylenol and Lysol contains para chloroorthobenzylphenol.
- Damage to nucleic acid: Ethylene oxide is a colorless gas which is soluble in water. It has alkylating action on proteins and damages the nucleic acid. Inhibition produced by it is irreversible, resulting in inhibition of enzyme activity. Formaldehyde gas acts on proteins by denaturation and on nucleic acids by alkylation of amino acid and sulfhydryl group of proteins and ring nitrogen atoms of purine bases. The reaction is irreversible. The 5’dGMP (deoxyguanosine monophosphate) interacts more rapidly with formaldehyde than 5’ GMP.
- Oxidation of sulfhydryl group of enzymes: Hydrogen peroxide acts by producing destructive hydroxyl free radicals that attack membrane lipid, DNA, and other cell components. Halogen compounds such as chlorine and chlorinated compounds and iodine compounds act as disinfectants. Inactivation of microorganisms with chlorine compounds depends on various mechanisms such as oxidation of sulfhydryl enzymes, ring chlorination of amino acids; loss of intracellular contents; decreased uptake of nutrients; inhibition of protein synthesis; decreased oxygen uptake; oxidation of respiratory components; decreased adenosine triphosphate production and depressed DNA synthesis. In other way, iodine compounds can penetrate the cell wall of microorganisms quickly, and result disruption of protein and nucleic acid structure and the synthesis of the microorganisms. Actually, the iodine compound inhibits protein synthesis and oxidizes –SH group of amino acids. Example: Povidone-iodine solution is used to rapidly kill (seconds to minutes) of S. aureus and M. chelonae at a 1: 100 dilution.
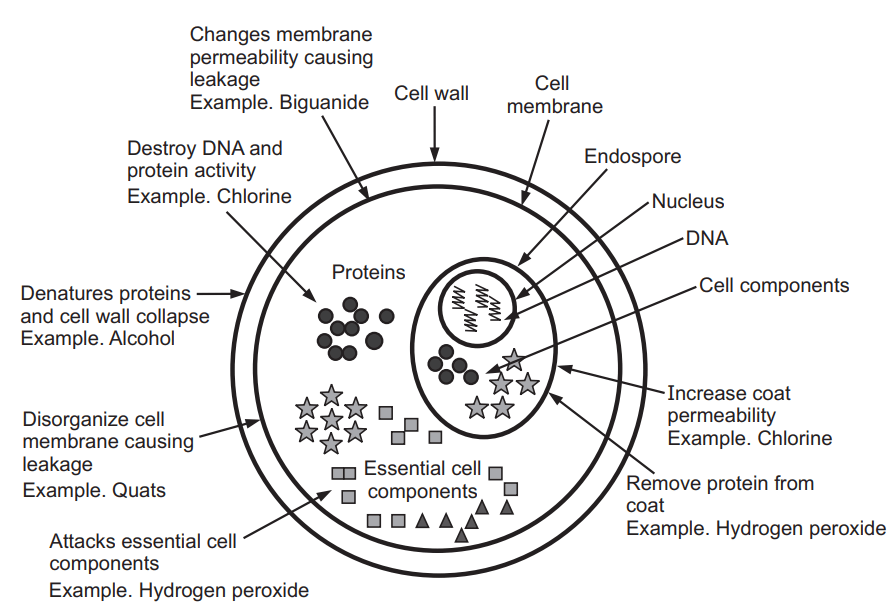
Mode of Action of Disinfectants
The main function of disinfectants is to act on microorganisms and the action is two ways either growth inhibition (bacteriostasis, fungistatic) or lethal action (bactericidal, fungicidal, or virucidal effects). But the main objective of the disinfectants is the lethal action or lethality.
Generally, disinfectants are either complex formulations of active molecules or contain co-solvents, chelating agents, acidic or alkaline agents, or surface-active products. Hence, these disinfectants are working on various mechanisms likely, acting on the membrane, leakage of the membrane, denaturation of cellular proteins, oxidation of –SH group of enzymes, Alkylation of amino, carboxyl, and hydroxyl groups, and nucleic acid damage. These activities are performed by various types of chemical agents which are acting as disinfectants. In below diagram showed the various compounds and their target locations in the cells by which disinfection activity is occurring (Fig. 3.11, Table 1.1).
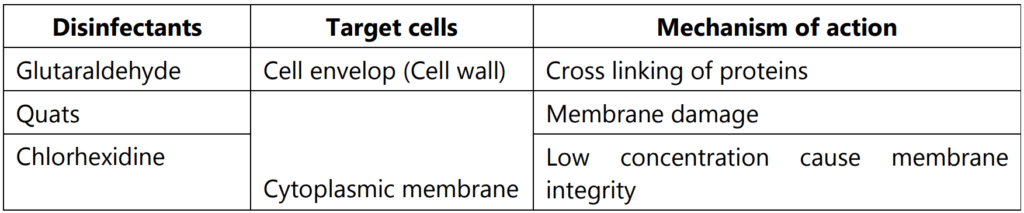
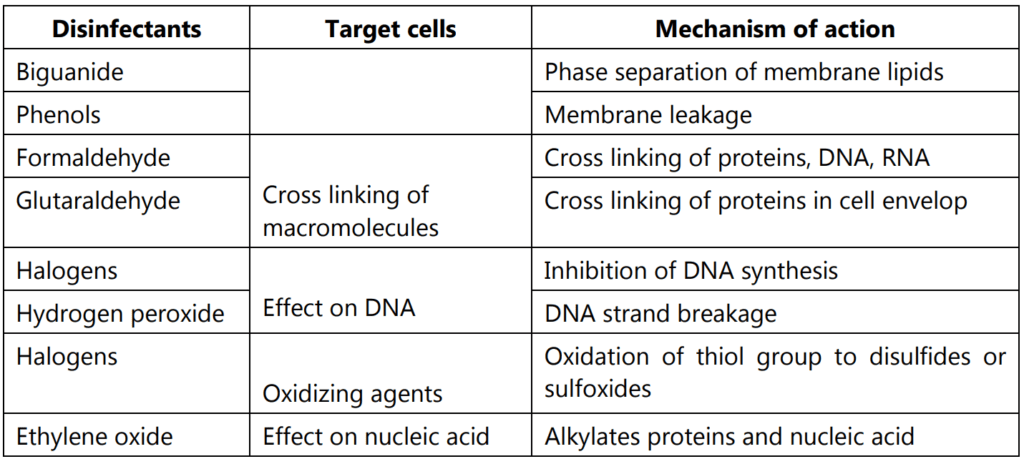
Different types of disinfectants are listed in Table 1.2 with their marketed products.
Make sure you also check our other amazing Article on : Fungi
