Like chromatography, electrophoresis is another very important technique which is used for the separation of constituents. It is also known as cataphoresis. The separation in electrophoresis is based on the different migration rates of charged ions in an electric field. The Swedish chemist in 1930 named Arne Tiselius performed the serum protein study by this technique and got a noble prize in 1948. Electrophoresis is used extensively for the separation of various types of constituents like Vitamins, Inorganic anions, cations, amine drugs, catechol, nucleic acid, polynucleotide, nucleotide, proteins, carbohydrates, drugs, etc.
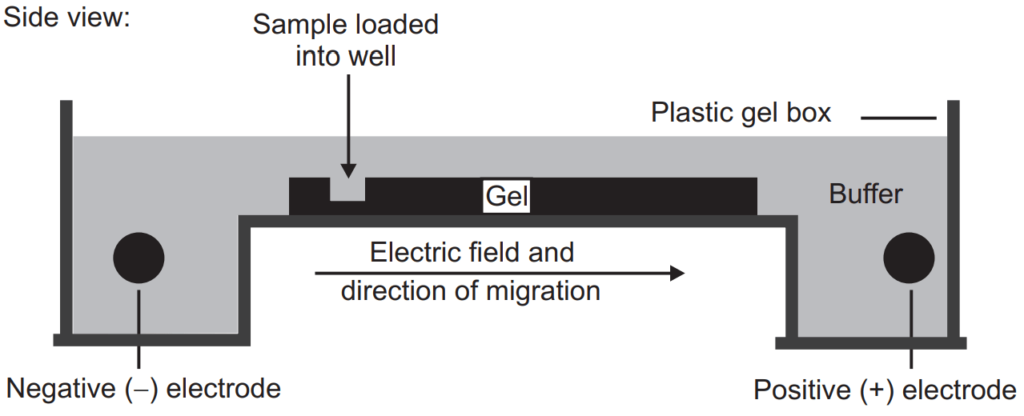
Electrophoresis is one of the most common tools for the separation of the protein (antibodies, enzymes, hormones) and nucleic acid (RNA and DNA). In the electrophoresis separation process, a small amount of sample is introduced (in the form of the band) into an aqueous buffer solution and applied high voltage, the entire length of a buffer with the help of a pair of electrodes (present at the end of buffer). Due to the field, the charged sample will move towards the electrodes (depending upon their charges) and the separation will take place.
The movement of the sample ions depends upon the different factors like size of the ions, the voltage applied the charge of ions, viscosity of the medium, ionic strength and pH of the buffer media, etc.
- Size of the ions: The mobility of the sample is inversely proportional to their size. It means the larger the size of the sample particle will give the lower the rate of separation.
- The voltage applied: If we have to obtain the sharp band we should apply a higher voltage which also speeds up the separation process. But due to high voltage, the problem of evaporation of solvent or buffer may arise.
- Charge of ions: Higher the charge on the ions the mobility of the sample will be higher or the rate of the separation will be faster.
- The viscosity of the medium: Mobility is proportional to the viscosity of the medium.
- Adsorption: Sample is retained over the supporting medium is known as adsorption which causes tailing of sample and thus reduces the resolution and rate of separation.
- Temperature: The migration time decreases with an increase in the temperature.
Table of Contents
Type of Electrophoresis:
Zone electrophoresis: In zone electrophoresis, the charged molecule or ion moves on the supporting media like gel, paper, etc. Examples of zone electrophoresis are:
- Paper electrophoresis
- Gel electrophoresis
- Thin layer electrophoresis
- Cellulose acetate electrophoresis
Moving boundary electrophoresis: In the moving boundary electrophoresis, the charged molecule can move freely in a free moving solution. There are no supporting media like gel or paper required. Examples are:
- Capillary electrophoresis
- Isotacto electrophoresis
- Isoelectric focusing
- Immuno electrophoresis
Paper Electrophoresis
This technique can be useful for the separation of amino acids, small proteins, or small charged molecules. The paper strips are moist with buffer and the end of this paper strip is dipped into buffer solution which contains electrodes. The sample should be applied in the center of the paper and the high voltage applied. The sample will migrate depending on their charges. After electrophoresis, the isolated constituents can be identified by various techniques of staining depending upon their chemical structure.
Gel Electrophoresis
Just like the other electrophoresis techniques it also involves the electric field. Those phytoconstituents which have to be separated are introduced into the gel under an electric field. The gel contains the pore through which phytoconstituents have to move. The small molecules of the phytoconstituents will travel a larger distance (because it can penetrate a small pore of the gel) through the gel compared to the larger molecules. Generally, the DNA and RNA (contain negative charge) can be easily separated by gel electrophoresis. If the protein has to be separated by this technique they are first treated with sodium dodecyl sulfate which unfolds the protein and makes them linear and coated with a negative charge. Currently, agarose gel or polyacrylamide gel are used for gel electrophoresis.
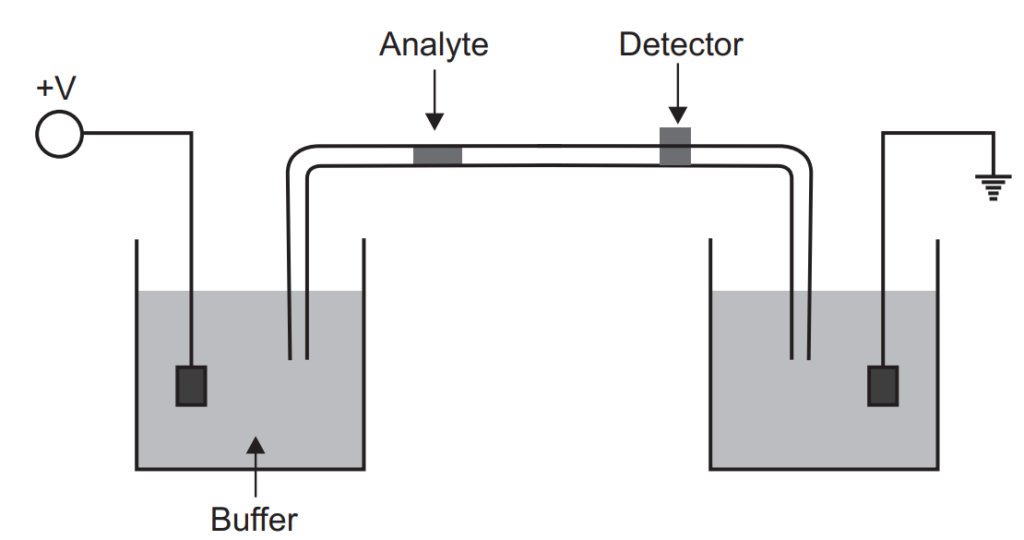
Capillary Electrophoresis
In the capillary electrophoresis separation takes place inside the capillary having an internal diameter of 10 to 100 µm. The ends of the fused silica capillary tube dip into the buffer solution. It contains platinum electrodes (cathode and anode). Capillary tubes contain an optical window for detection which is attached to a UV detector. To introduce the sample the inlet buffer should be replaced with sample and the sample can be injected by pressure, electrokinetically, capillary action, and siphoning. On applying the voltage the migration of the sample starts which is known as electrophoretic migration but this electrophoretic migration is also affected by the electron osmotic flow of the buffer solution. Generally, the electroosmotic flow is towards the cathode (or negative charged). Though the electrophoretic mobility is lower than electro-osmotic flow all the analytes are carried towards the cathode at a different speed. These can be detected by the detector.
Make sure you also check our other amazing Article on : Uv Spectroscopy
