Aim: Steam distillation:- To calculate the efficiency of steam distillation
Requirements:
Chemicals: Turpentine Oil.
Apparatus/ Instruments: Distillation Flask, Steam Generator, Condenser, Separating Funnel, Double Holed Rubber Cork, Measuring Jar, Thermometer, Bent tubes, Weighing Balance/Weights, etc.
Principle of steam distillation
Steam distillation is a process used for the separation of high boiling point substances from non-volatile impurities. An important application of Steam distillation is high boiling point liquids can be distilled well below their boiling points. It is the way of separating miscible liquids based on their volatilities. The boiling point of products is minimized, permitting the constituents to be vaporized. The efficiency of Steam distillation is more effective than simple distillation. Steam distillation is especially used for the separation of volatile oils at low temperatures without decomposition.
Applications of steam distillation
- It is used in the isolation of essential oils, for use in perfumes.
- It is also useful to separate intermediate or final products during the synthesis of complex compounds.
- It is widely used in petroleum refineries and petrochemical plants commonly referred to as steam stripping.
- It is also important in the separation of fatty acids.
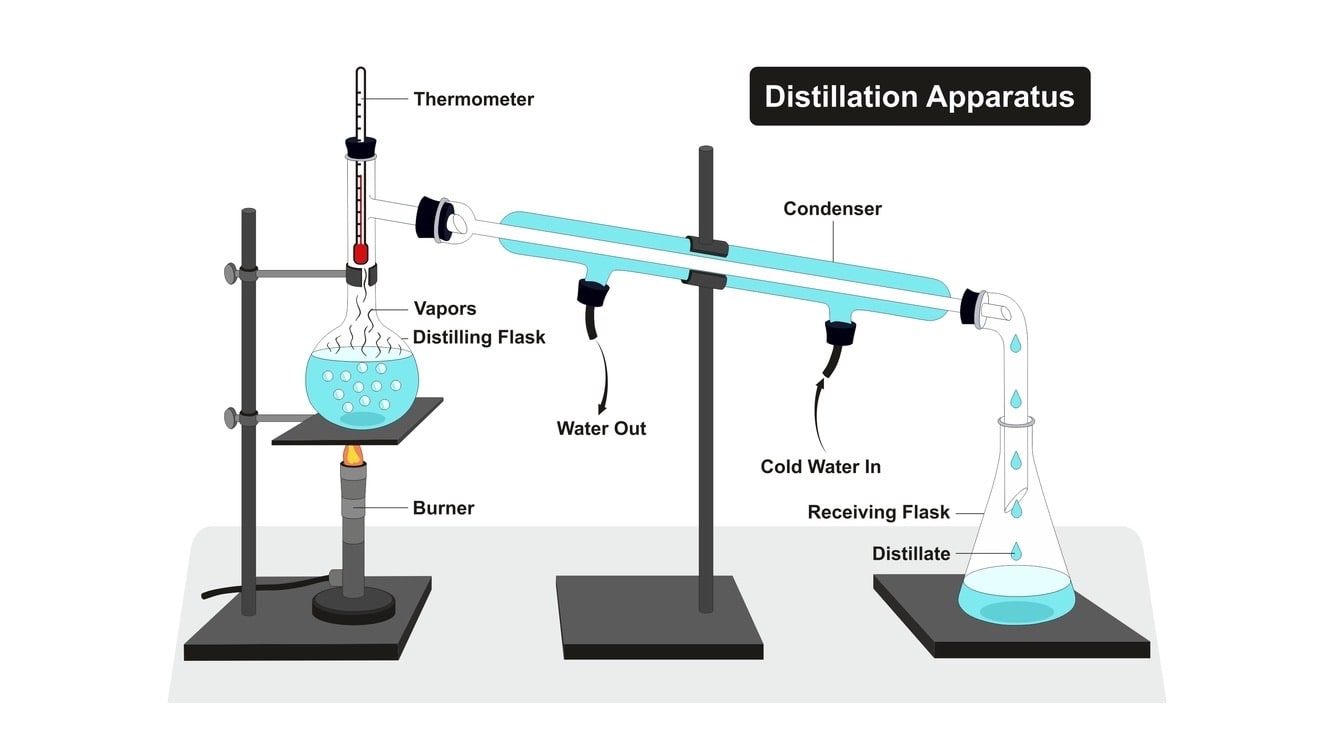
Procedure:
- 30ml of turpentine oil and 70ml of water is measured and transferred into a distillation flask.
- The flask is closed with a two-holed rubber cork and a thermometer is inserted through one of the holes and fixed.
- Through the other hole, a bent tube of steam generator for the steam is fixed to pass into the distillation flask.
- Ensured the bent tube is reach almost near the bottom of the flask but not touching the flask.
- The side tube of the neck of the distillation flask is fixed to the condenser.
- Further, the steam is allowed to pass into the distillation flask continuously from the steam generator.
- The mixture gets heated. Ager some time it starts boiling.
- The temperature T1 at which boiling occurs is noted.
- Steam carries the vapor of oil and passes it into the condenser where condensation takes place.
- Condensate is collected into the receiver for 15 minutes.
- Oil is separated from water using a separating funnel and the volume /weight of oil and water is noted.
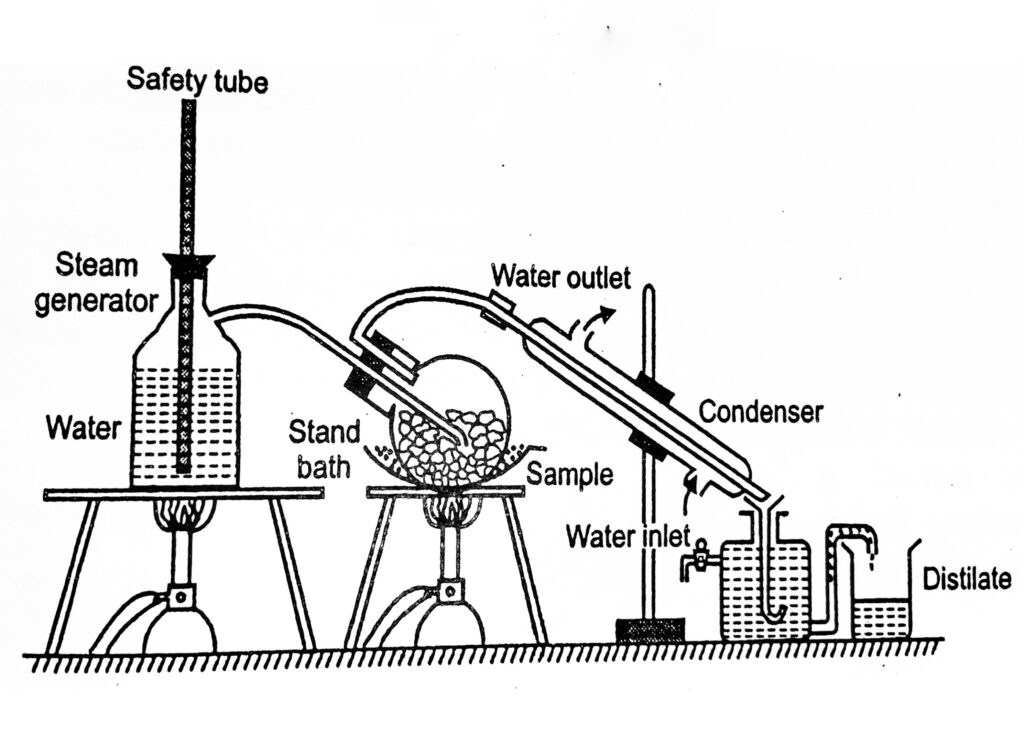
Observations And Calculations:
The molecular weight of water: 18.016 (MW)
The molecular weight of turpentine oil: is 276.283 (Mt)
The boiling point of the mixture starts: at 95.6 0 C
At 95.6 0 C vapor pressure of water: 647 mm of mercury (Pa)
Atmospheric pressure: 760 mm of mercury
Vapour pressure of turpentine oil: Atmospheric pressure- vapor pressure of water at 95.6 o C
760 – 647 = 113mm of mercury (Pb)
The volume of the water in distillate: =
The volume of the turpentine oil in distillate: =
Density of water = 1 g/ml at 25o C
The density of the turpentine oil = 0.86 g/ml at 25 o C
Weight of water ager distillation (Wa) = volume of water X density of water = ——– gm
Weight of turpentine oil ager distillation (Wb) = volume of oil X density of oil = ——– gm
Practical yield = Wb /Wa
Theoretical yield:
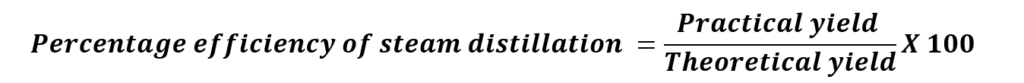
RESULTS & DISCUSSION:
Percentage efficiency of steam distillation =
Make sure you also check our other amazing Article on: Effect of Time on Rate of Crystallization