Electrophilic Substitution in Naphthalene: The aromatic compounds like naphthalene, anthracene and phenanthrene are called polycyclic aromatic compounds because they contain two or more rings fused together containing one or more common carbon bonds. These compounds are cyclic, contain (4n + 2) π pi electrons, and are planar. Every carbon in the ring is sp2 hybridized. Naphthalene is aromatic (resonance energy 61 kcal/mole) and is similar to benzene in reactions.
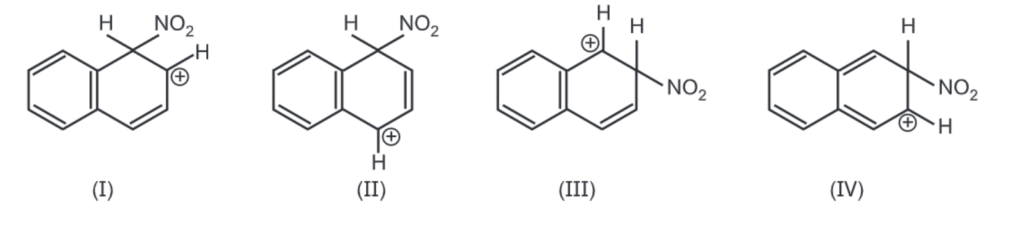
Nitration and halogenation occur almost selectively in the α-position. The transition states for both the attack (α) and (β) have four important resonating structures as follows:
The carbocation generated by the attack of nitronium ion at a-position is a hybrid of structures (1) and (II). In both these structures, the aromatic sextet is preserved. Attack at B-position generates the carbocation that is a hybrid of (III) and (IV). Here only structure (III) has an aromatic nature. Therefore, the overall energy required to attain the transition states for α-attack is less than that of β-attack. Hence nitration would occur much more rapidly at the α-position.
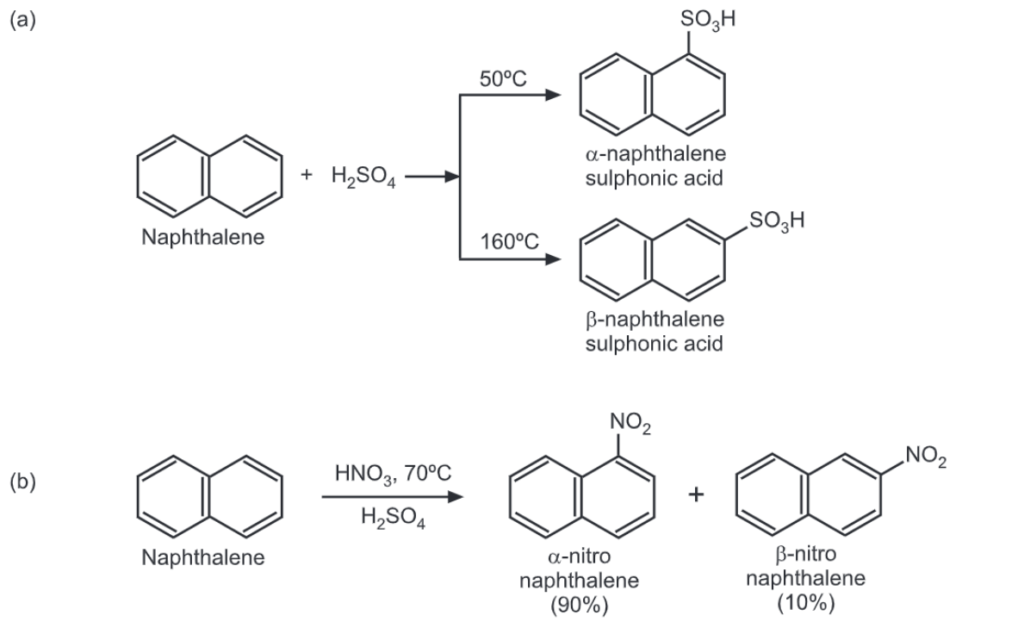
In the case of the transition state of naphthalene, at least one ring retains its aromatic character. Since, this is not possible in benzene, the energy of the transition state for naphthalene is less than that for benzene by about 10 kcal/mole. Hence, naphthalene in general is more reactive than benzene. For example, bromination of naphthalene occurs in absence of the catalyst. Upon nitration by nitric acid in acetic acid, naphthalene gives mainly 1-nitronaphthalene while thiophene gives 2-nitrothiophen.
Make sure you also check our other amazing Article on : Theory of Orientation