Phenanthrene is the resonance hybrid of the following 5 resonating structures.
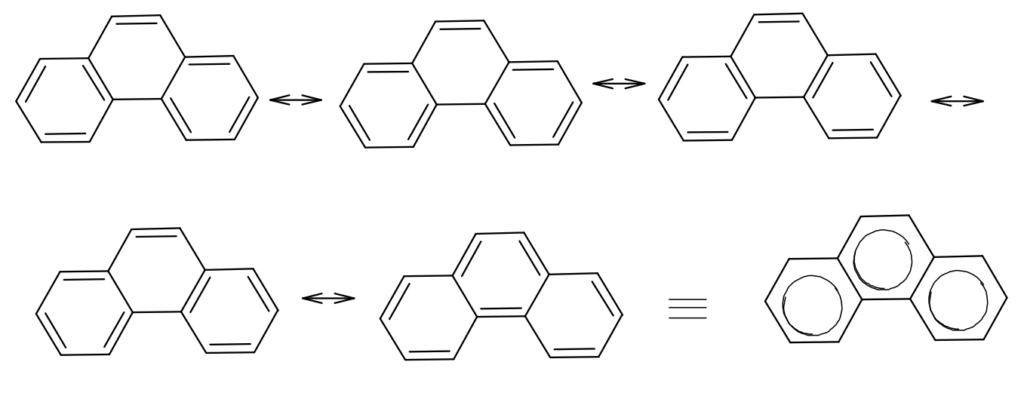
The resonance energy for phenanthrene is 92 Kcal/mol, that for anthracene is 84 Kcal/mol and for naphthalene and benzene rings are 61 and 36 Kcal/mol respectively. So in phenanthrene, the resonance energy of each benzene ring is 30.3 Kcal/mole and that’s why phenanthrene is more stable than anthracene but less stable than benzene.
In phenanthrene, the C9 – C10 bond is shorter than the C1 – C2 bond due to more double bond characters.
There are 5 monosubstitution products: 1, 2, 3, 4, and 9.
Reactions of Phenanthrene
Phenanthrene is reactive at 9, & 10- positions.
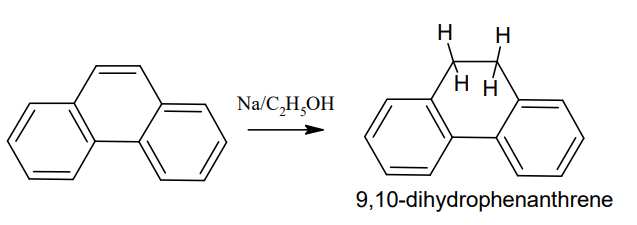
Reduction: Phenanthrene is catalytically reduced to 9,10-dihydrophenanthrene using
sodium in ethanol (Addition reaction)
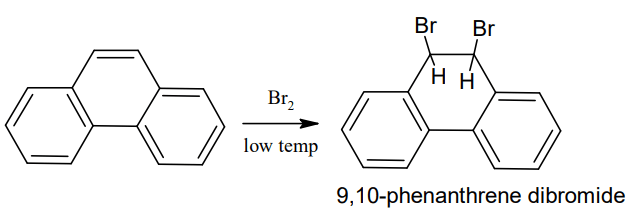
Halogenation: It adds on bromine to form 9,10-dibromophenanthrene (addition reaction)
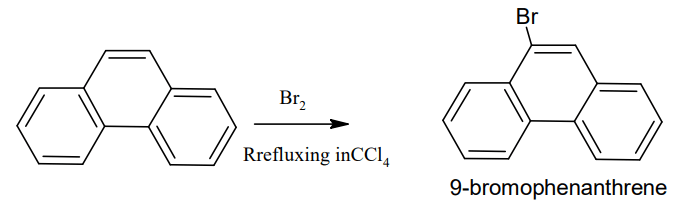
But when refluxed phenanthrene solution in CCl4 with bromine at a high temperature it
give 9-bromophenanthrene (Electrophilic substitution)
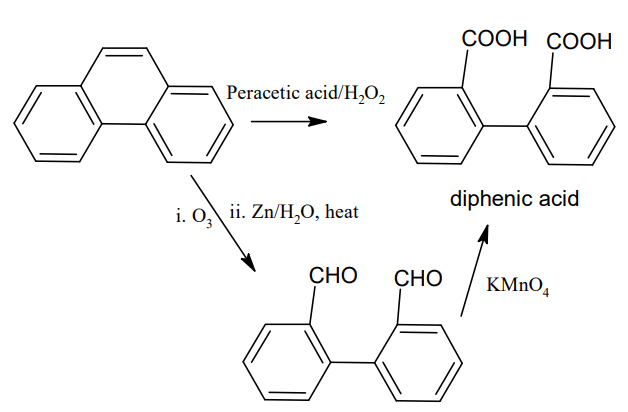
On oxidation with peracetic acid or on ozonolysis followed by oxidation phenanthrene
gives diphenic acid
Uses of Phenanthrene and its derivatives
- Phenanthrene can be used in the manufacture of pesticides and dyes
- After conversion processing, it can be used to produce dyes, drugs, and resins
- It is used as a stabilizer. the high efficiency and low toxicity pesticides and smoke-less powder explosives.
- Phenanthrene quinone can be used as dyes, fungicides, and polymerization inhibitors.
- The 9,10-biphenyl dicarboxylic acid derivative is used to manufacture polyester and alkyd resin.
Make sure you also check our other amazing Article on : Haworth Synthesis of Anthracene
