What is absorption explained with an example?
Absorption is the transfer of the drug from its site of administration to the bloodstream. The rate and extent of absorption depend on the route of administration, the formulation and chemical properties of the drug, and physiological factors which can impact the site of absorption. It is illustrated in Fig. 1.1. When a drug is administered intravenously, the entire dose is available in the systemic circulation. Administration of a drug by any other route may result in less availability of the drug due to incomplete absorption. When a tablet or capsule is swallowed, it must dissolve before its absorption. The process is called dissolution. Once dissolution has occurred, the drug molecules pass through the membrane of the cells lining the gastrointestinal tract to reach the bloodstream. The drug will be absorbed by one of the mechanisms mentioned earlier. The rate and extent of absorption of a drug are termed as its bioavailability.
Drug Bioavailability
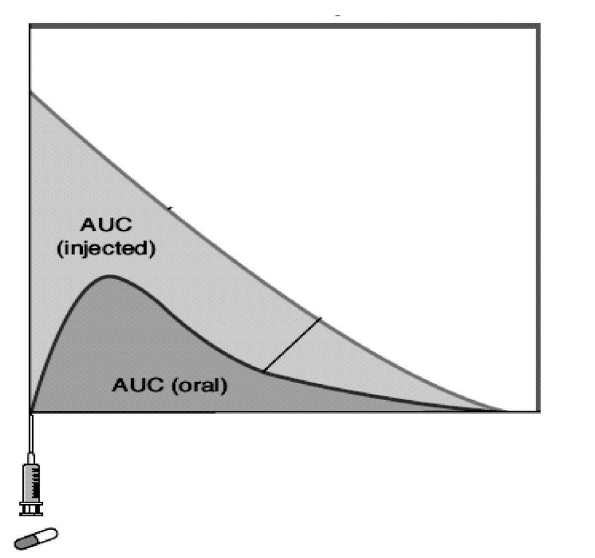
When a graph of plasma concentration of a drug is plotted against time, one gets a curve as depicted in Fig. 1.1. The area under the curve (AUC) of a plasma concentration versus time represents the total amount of drug reaching the systemic circulation. AUC will have a different shape depending on the route of administration. The AUC obtained after intravenous administration is considered to be 100%. This is termed as the absolute bioavailability of a drug. Any other route (intramuscular, subcutaneous, oral, dermal, etc.) will have lesser bioavailability. The ratio of AUC for any formulation/ route of administration in comparison to intravenous formulation is termed as the relative bioavailability of a drug.
The acid environment or presence of food in the stomach, the solubility and other chemical properties of the drug, and the effect of initial exposure to metabolic processes in the liver may reduce the amount of drug which reaches the systemic circulation after oral administration, thereby reducing the bioavailability of the drug. If the drug is subject to metabolism by the liver, the amount of drug reaching the systemic circulation is decreased; e.g. propranolol, enalapril. As a result, the substantial drug is lost due to metabolism during a single passage through the liver. This is called as the first-pass effect. When the drugs are highly susceptible to the first pass effect, the oral dose needed to cause a response will be significantly higher than the intravenous dose used to cause the same response.
Bioavailability becomes an important parameter in drug-product selection. While two generic products may contain the same active ingredients, they may not have the same dissolution or absorption characteristics. Hence, they cannot be considered bioequivalent. In the case of extended-release products, the change in dissolution characteristics is intentional. In some cases, products are poorly manufactured and should be avoided. Generic equivalent products approved by Food and Drug Administration (FDA) must meet a standard of less than 20% variation from the comparison product. Bioequivalency data is published by the USFDA’s Centre for Drug Evaluation and Research, referred to as the “Orange Book”, and is available on the web.
Factors affecting drug absorption
A number of patient-specific factors can be affecting drug absorption. Proper absorption needs adequate blood flow to the site of administration. Most absorption after oral administration occurs in the small intestine because of a larger surface area and greater blood flow. If the small intestine is partially removed, as in the case of bariatric surgery, impairment of absorption of drugs can occur.
Contact time with the epithelial lining of the gastrointestinal tract is an important factor in drug absorption. In patients with severe diarrhea, drug absorption may be adversely affected because of rapid transit time in the gastrointestinal tract. Contrary to this, if gastric emptying time is delayed, as in the case of a large fatty meal, it may delay and potentially reduce absorption. Some medications exhibit drug-food or drug-drug interactions with other compounds present in the gastrointestinal tract. The interaction between tetracycline and dairy products or antacids is an example. The absence of hydrochloric acid in the stomach is called as achlorhydria. It is common in the elderly population. Patients with achlorhydria may experience inadequate tablet dissolution and therefore poor drug absorption, it’s a factor affecting drug absorption.
Make sure you also check our other amazing Article on: Membrane Transport Types