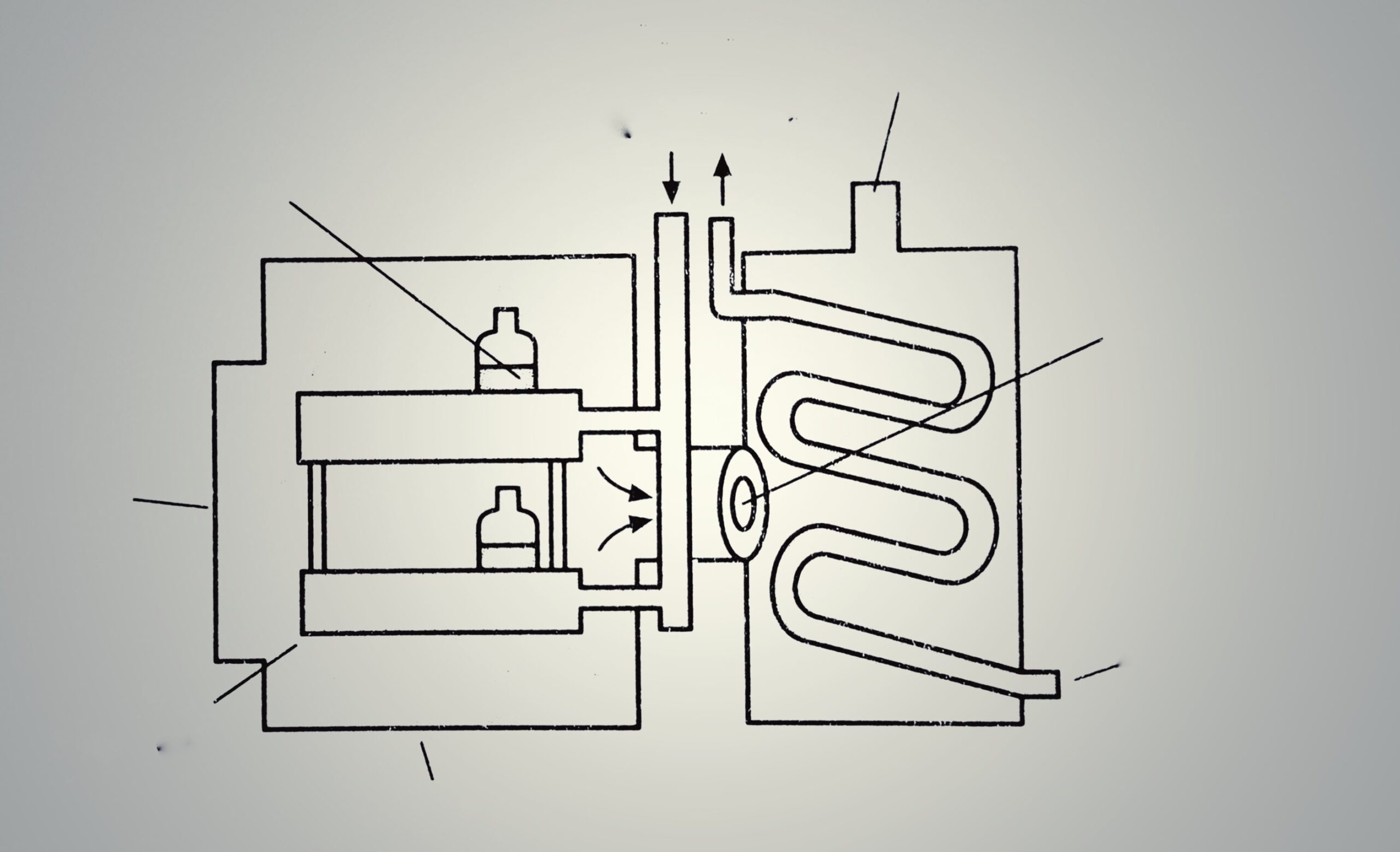
Freeze dryer is also known as lyophilization, i.e., the system is made solvent loving for removing the same.
Table of Contents
Principle of the Freeze dryer
In freeze-drying, water is removed from the frozen state by sublimation, i.e., direct change of water from solid into vapor without conversion to a liquid phase. Solid-liquid-vapour equilibrium. phase diagram of water is useful to decide the experimental conditions. The drying is achieved by subjecting the material to tempera and pressures below the triple point (in practice, the below eutectic temperature is essential). Under these conditions, any heat transferred is used as latent heat, and ice sublimes directly into the vapor state. The water vapor is removed from the system by condensation in a cold trap maintained at a temperature lower than the frozen material.
Construction of the Freeze dryer
The construction of a freeze dryer is shown in Figure 1.1. It consists of:
- Drying chamber in which trays are loaded.
- Heat supply in the form of the radiation source, heating coils.
- Vapour condensing or adsorption system.
- Vacuum pump or steam ejector or both.
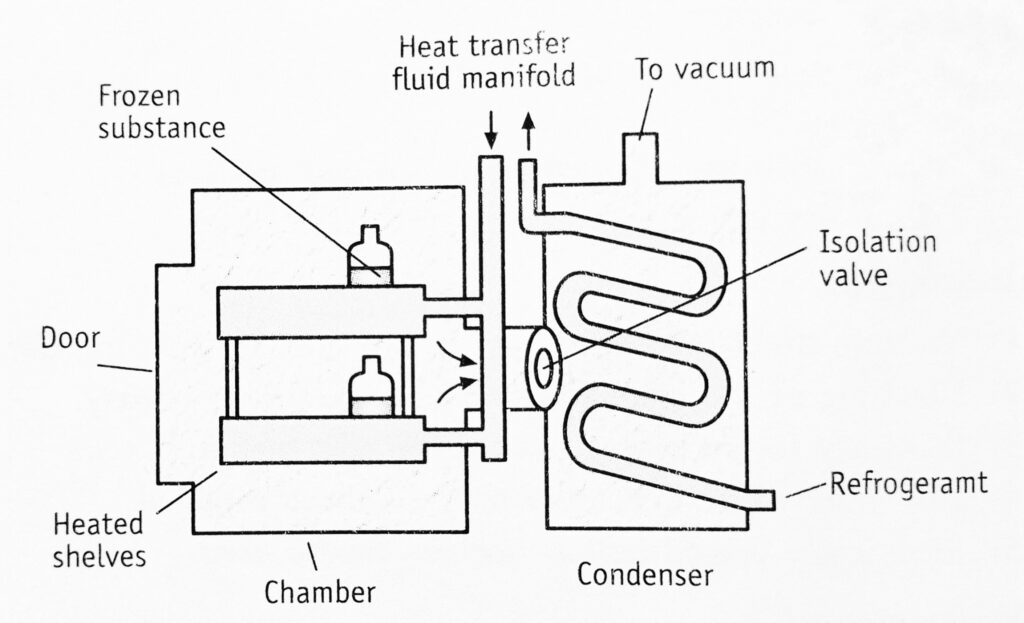
The chamber for vacuum drying is generally designed for batch operation. It consists of shelves for keeping the material. The distance between subliming surface and condenser must be less than the mean path of molecules. This increases the rate of drying. The condenser consists of a relatively large surface cooled by solid carbon dioxide slurred with acetone or ethanol. The temperature of the condenser must be much lower than the evaporated surface of the frozen substance. In order to maintain this condition, the condenser surface is cleaned repeatedly.
Working on the Freeze dryer
The working of the freeze dryer consists of the following steps.
- Preparation and pretreatment
- Pre freezing to solidify water
- Primary drying (sublimation of ice under vacuum)
- Secondary drying (removal of residual moisture under high vacuum)
- Packing
Preparation and pretreatment: The volume of solution introduced into the container is limited by its capacity. Satisfactory freeze-drying beyond a certain limit of the depth of liquid is not possible. Therefore pretreatment is essential. The solution is pre-concentrated under normal vacuum tray drying. This reduces the actual drying by 8 to 10 times. The final product becomes more porous. Liquid or solid desiccants are also used for this purpose.
Pre freezing to solidify water: Vials, ampoules, or bottles in which the aqueous solution is packed are frozen on cold shelves (about -50 °C). During this stage, the cabinet is maintained at low temperature and atmospheric pressure. The normal cooling rate is about 1 to 3 Kelvin per minute so that large ice crystals with relatively large holes are formed on the sublimation of ice. This is also responsible for giving a porous product.
Primary drying (sublimation of ice under vacuum): In this step, the material to be dried is spread on as large a surface as possible for sublimation. The temperature and pressure should be below the triple point of water, i.e., 0.0098 °C and 0.533 kilopascals. (4.58 mmHg) for the sublimation, when water alone is present.
When a solution of solid is dried, the depression of the freezing point of water occurs. Hence, it is essential that the temperature be brought below the eutectic point. The pressure and temperature at which the frozen solid vaporizes without conversion to a liquid are referred to as the eutectic point. Depending on the drug substance dissolved in water, the eutectic point is determined. The usual range is from -10 °C to 30 °C. The condition of 1 to 8 K below the eutectic point is sufficient.
Vacuum is applied to the tune of about 3 mmHg (0.4 kilopascals) on the frozen sample. The temperature is linearly increased to about 30 °C in a span of 2 hours.
Heat (about 2900 kilojoules per kg) is supplied which transfers as latent heat and ice sublimes directly into a vapor state. The heat controls. the movements of the ice layer inwards. It has to be controlled in such a manner so as to get the highest possible water vapor at the ice surface without melting the material. As soon as vapor molecules are formed, these are removed. The overall driving force is the temperature difference (also vapor pressure difference) between evaporating surface and the condenser.
As the drying proceeds, the thickness of the frozen layer decreases, and the thickness of partially dried solids increases. The primary drying stage removes easily removable moisture. During this stage, about 98% to 99% of water is removed. Still, traces of moisture is present in the sample.
Secondary drying (Removal of residual moisture under high vacuum): During this stage, traces of moisture is removed. The temperature of the solid is raised to as high as 50 to 60 °C, but the vacuum is lowered below that is used in primary drying (50 mmHg). The rate of drying is very low and it takes about 10 to 20 hours.
Packing: After the vacuum is replaced by an inert gas, the bottles and vials are closed. Uses Freeze dryer is most commonly used in the production of dosage forms, such as injections, solutions, and suspensions. It is used. for drying of a number of products.
- Blood plasma and its fractionated products. Bacterial and viral cultures.
- Human tissue (arteries and corneal tissue). Antibiotics and plant extracts.
- Steroids, vitamins, and enzymes.
Several other products such as food items (prawns, mushrooms, meat, and poultry products), coffee and tea concentrate, and citrus fruit juices are dried.
Advantages:
The entire operation is carried out well below the freezing point. This offers several advantages.
- Thermolabile materials (heat-sensitive materials) can be dried.
- The product retains its bulk volume. It is porous and uniform. The reconstitution of the material is easy.
- Denaturation does not occur.
- Migration of salts and other solutes does not take place.
- Loss of volatile material is less.
- Moisture levels can be kept as low as possible without decomposition.
- Material can be dried in its final containers such as single-dose and multiple-dose vials.
- Sterility can be maintained.
- The final product can be stored at ambient temperature, if well sealed by providing an inert atmosphere.
Disadvantages:
- The product is prone to oxidation, due to its high porosity and large surface area. Therefore, the product should be packed in a vacuum using inert gas or in a container impervious to gases.
- Equipment and running costs are high.
- It is difficult to adopt the method for solutions containing non-aqueous solvents.
- The period of drying is high (rarely less than 10 hours). The time I cannot be shortened.
Make sure you also check our other amazing Article on : Vacuum Dryer