Gas chromatography is generally divided into GSC (gas-solid chromatography) and GLC (gas-liquid chromatography). In either type, the gas is a mobile phase but the stationary phase varies in GC (gas chromatography). In GLC stationary phase is liquid and in GSC it is solid. In GLC the principle of separation is partition while in GSC it is adsorption. Most of the time when named GC it is GLC.
Generally, a liquid is coated on solid support used as a stationary phase. The mixture which has to separate into the individual constituent has to be converted into the vapor and mixed with the mobile phase (gas). The constituent which has more affinity towards the stationary phase travels slowly compare to those which have a high affinity towards the mobile phase. The constituents are separated out on the basis of their partition coefficient. GLC is a good technique to detect those compounds which are volatile and thermostable.
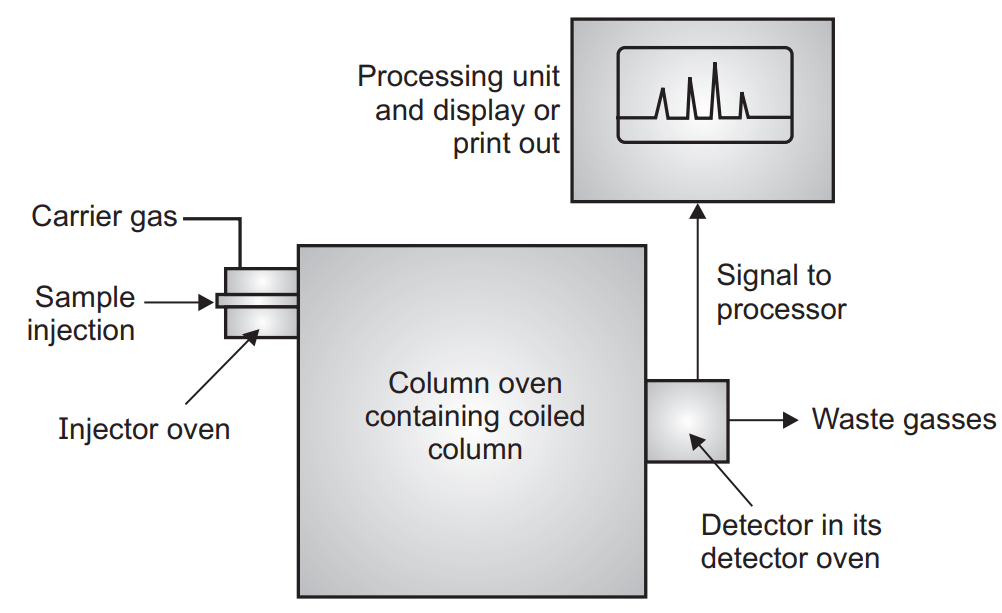
The volatile compound should be mixed with the carrier gas. The carrier gas may be hydrogen, helium, nitrogen. Hydrogen is a good option for carrier gas because of its good thermal conductivity and low density but it reacts with unsaturated compounds. Helium is also a good choice but it is expensive. Nitrogen is inexpensive but has reduced sensitivity. Gases are generally stored under high pressure. To blow the gases under uniform pressure and flow rate there is a need for a flow meter. Generally, rotameter and soap bubble meters are used to control the flow of gases.
The sample can be introduced into any form like solid, liquid, or gaseous form. Valves are a suitable device to introduce the gas sample. Solid samples are generally dissolved in an appropriate solvent and then injected through the septum. Liquid samples can be dispensed through either loop or septum devices. Septum should be made of high-quality silicone rubber and can tolerate high temperature and is suitable for repeated injection.
Another important part of GLC which effect the separation of the constituent is a column. They are made of either glass or stainless steel. The stainless steel column has a long life and is easy to handle but sometimes it may react to the constituent which is not in the case of glass columns but glass columns are fragile and difficult to handle. The column may be analytical (length 1-1.5 mt, diameter 3-6 mm) or preparative column (length 3-6 mt, outer diameter 6-9 mm). Depending upon its nature it may be a packed column, open tubular or Golay or capillary column and support coated open tubular column (SCOT).
Though the sample should be converted into vapor the pre-heaters are required in the GLC(gas-liquid chromatography) which converts the sample into vapor form and mix with the carrier gas. They are installed along with injecting device. A thermostatically controlled oven can be used for this purpose which can operate on an isothermal programming base (same temperature during entire operation) and linear programming (oven is heated linearly over a period of time).
Different type of detector like kathrometer, FID (flame ionization detector), AID (argon ionization detector), ECD (electron captured detector) are used. The most sensitive of them are ECD (10-12). Recorders record the response and amplify it. Recorder record the retention time, record base line, and record all the peaks. Heights, width, area of the individual peak, percentage of area are calculated by integrators.
The separation or detection of the sample can be improved in GLC(gas liquid chromatography) by derivatization techniques. It can be precolumn derivatization or post column derivatization. In precolumn derivatization, the sample is converted into a more volatile and thermostable derivative (like carboxylic acid, phenols, sugars are converted into less polar by reagent BSA bis trimethyl silyl acetamide). Post column derivatization is done generally to improve the detector response for isolated constituents.
GLC(gas liquid chromatography) can be used for the qualitative analysis by measuring the retention time of the sample under standard conditions but generally, it is used for quantitative analysis by direct comparison method, calibration curve method, and by internal standard method.
Make sure you also check our other amazing Article on : High Pressure Liquid Chromatography
