Halogenation is accomplished by treating the aromatic compound with molecular halogens like Cl₂, Br₂ or I2 Fluorination is highly exothermic and explosive. Chlorination and bromination have moderate speed and are easy to carry out. While iodination is a slower reaction and is possible only with activated aromatic compounds like phenols, aniline, etc.
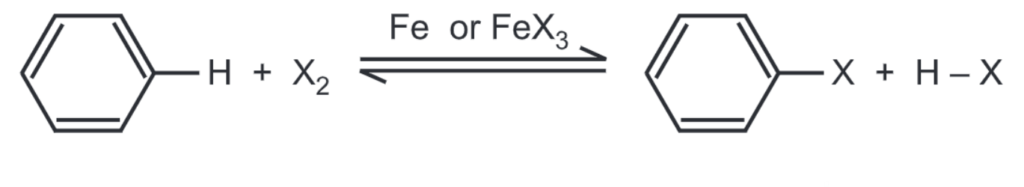
To speed up the reaction usually iron metal, or ferric halide is added in catalytic amounts.
Certain Lewis acids such as AICI3, or AlBr3 are also effective catalysts. The Lewis acid polarises the halogen molecule to promote interaction between the positively charged electrophilic end of the halogen molecule and the π-cloud of benzene.
The general mechanism of halogenation includes
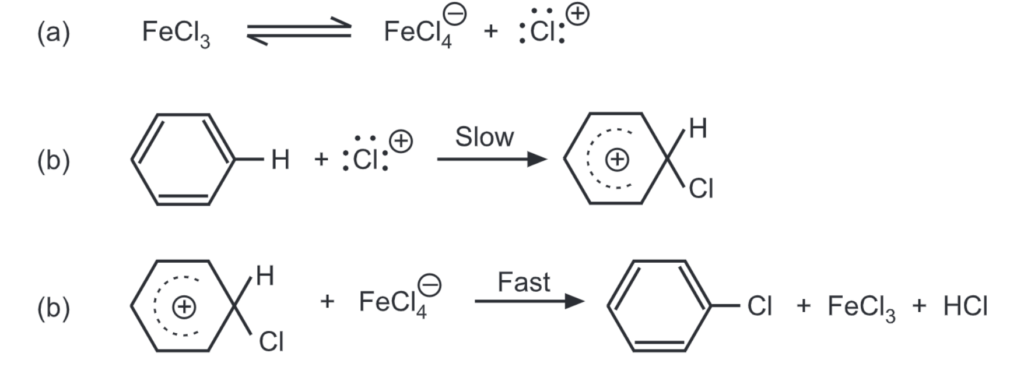
The iron atom in FeCl3 is electron deficient. It may complete its octet by accepting a pair of electrons from a chlorine atom and acquiring a negative charge. An electron-deficient: Cl ion is generated as a result, which attacks the aromatic ring. Halogenation may also be carried out by:
- The use of interhalogen compounds like Br – Cl; I- Cl so that the attack of the л-electron cloud will occur through the less electronegative halogen atom.
- Hypo-halous acid HO-X too. This reaction is slower than with molecular halogens, X_{2} because OH is a poorer leaving group from HO-σ – X+σ than X – from X-X.
- In the presence of strong acid, however, H-OX becomes a very powerful halogenating agent due to the formation of a highly polarised complex as follows:
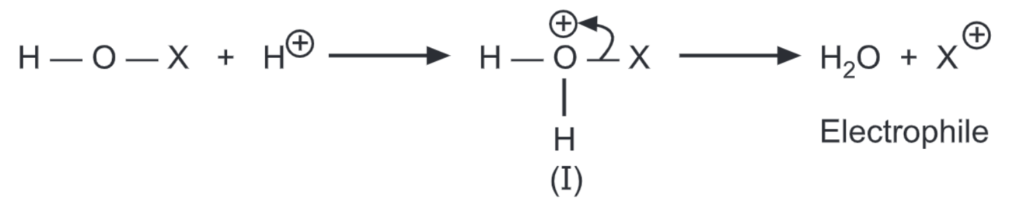
The species (I) is an effective electrophile and does not get further converted into water and X+ because H2O is a much better-leaving group than Cl–. Thus, (HOCl + acid) is a more effective chlorinating agent than Cl₂/AICl3.
Make sure you also check our other amazing Article on : Sulfonation of Benzene