In contrast to the column chromatography (like GLC, HPLC) planar chromatography utilizes a planar (flat) stationary phase for the separation. High Performance Thin Layer Chromatography they are:
Table of Contents
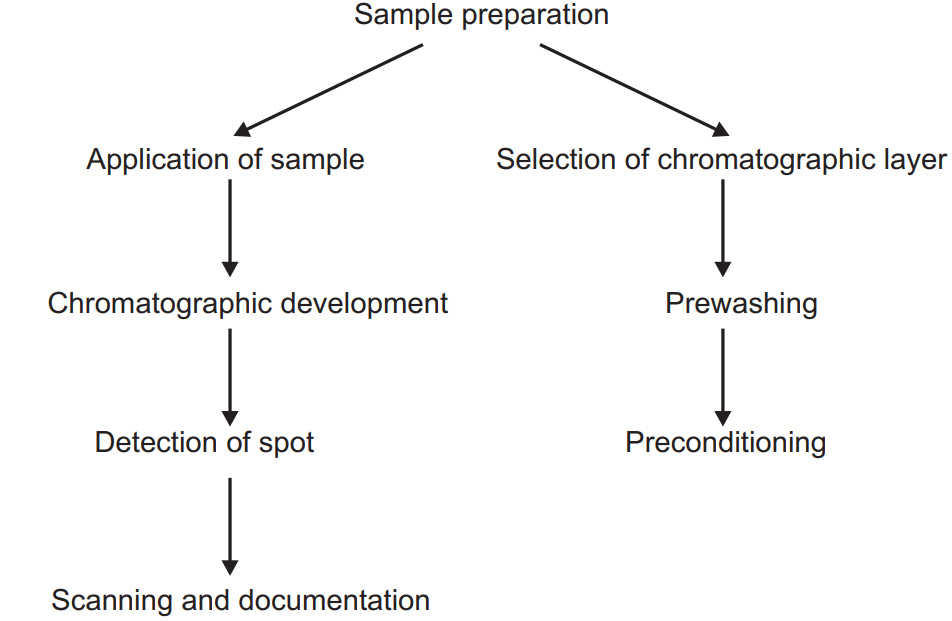
Sample Preparation
The sample should be homogenous and representative of the entire batch. The sample should be prepared such as that analyte present in the traces quantity can be detected. If the sample is pure and concentrated can be directly applied to the HPTLC plate but if the analyte is present at less concentration with impurities than purification, isolation of the analyte from the interfering reagent and concentration procedure should be taken.
Sample Application
The physical dimension of the isolated sample should be very much compact. The optimum amount of the sample should be applied to the stationary phase. In the case of manual application, care should be taken that manual application does not damage the surface of the layer. During sample application, chemical fumes and vapours should be absent and temperature and humidity should be constant. The fully automatic sample application is better. It dispenses precise volume on precise position with precise delivery rate. Generally, 0.5-5µl sample applied in case of spot or 2-10µl as narrow band. Sample can be applied as
- Manual application
- Instrumental techniques
- Semiautomatic application.
The adverse effect of overloading of samples shows unresolved components.
(HPTLC)
Stationary phase, TLC plates and solvents
Silica gel is the most popular solvent used in HPTLC afterwards cellulose. Homemade sheets/plates show bigger particle and are not shows homogenous so it is not suitable for HPTLC analysis. Precoated HPTLC plates which are available in the market are more appropriate for HPTLC analysis. Plastic sheet or aluminium foil supported HPTLC plates are now more popular compared to the glass plate supported HPTLC plate because they can easily cut into desired sizes as well as require less space to keep in the lab but glass is more resistant to heat and chemical reaction compared to the aluminium and plastic sheets. The mean particle size in HPTLC is 5-6µm compare to the 10-12µm in classical TLC. The layer thickness is 100-200µm in HPTLC but 250 µm in TLC. The relative humidity plays a crucial role to reproduce the result. The relative humidity is variable in the laboratory condition. To avoid such variation it is desirable to precondition (saturate the TLC chamber with the vapours of the mobile phase) the TLC chamber. Preconditioning is more required with the highly polar mobile phase.
The environment of the lab contains various dirt particles, the vapour of various gases which can be deposited into the HPTLC plate. This impurity can be removed by prewashing of the plate. Run the methanol or such other solvent without applying the sample on the HPTLC plate so that all dirt particles or impurities on the HPTLC plate will be collected on the upper edge which can be removed. After prewashing the plate are kept in the oven for 15-20 min at 120°C which is known as conditioning.
Mobile Phase:
The mobile phase used for HPTLC should be of utmost pure. The presence of antioxidants and stabilizers altered the nature of the chemical. They should be kept in proper storage condition. Polarity, viscosity, volatility are some points which affect the chromatographic procedure. The solvent used for the mobile phase is categorized into 8 different classes. The mobile phase should be kept as simple as possible.
Chromatographic Development:
Chromatographic development is another important aspect of HPTLC. Generally, the glass development chambers are used for such purposes. The various development chambers which can be used for chromatographic development are:
- Flat bottom chamber
- Twin trough chamber
- Sandwich chamber
- Horizontal chamber
- Automatic development chamber
- Forced flow development chamber
- Automatic multiple development chamber.
The twin trough chamber uses less solvent. The linear development of the chromatogram is the best method in which the HPTLC plate is placed vertically in an appropriate chamber. The solvent is run by capillary action.
Detection:
After the development of chromatogram HPTLC plates are dried and evaluated for the following method:
Evaluation by the non-destructive method:
- Direct visual method
- Evaluation under UV light:
Reversible Reaction:
- Iodine Vapour
- Ammonia Vapour
Non Reversible Reaction:
- Fluorescent dye
- pH indicator
- Wetting/ Dipping
- Spraying technique
All the above techniques come under a non-reversible detection technique in which the isolated constituents cannot be recovered and destroyed after detection.
Scanning and Documentation:
After the development of the spot, the HPTLC plate is scanned at selected UV regions wavelength and the selected can be measured in the computer in the form of peak and can be compared with standard compound or other constituents.
The obtained band can be converted into the peak. The height and area peak of the peak corresponding to the concentration of isolated constituents. This document can be stored on the computer for the further reference.
Make sure you also check our other amazing Article on : Gas Liquid Chromatography