Inflammation is a critical homeostatic process that is activated by cellular injury regardless of the mechanism of that injury. Inflammation is essentially local in nature, although cellular mediators released during inflammation may initiate systemic responses as well. Systemic inflammatory response syndrome is the most extreme form of inflammatory response and may be life-threatening in critically ill patients. This syndrome nearly always occurs in the setting of systemic infection and is termed sepsis.
Inflammation is defined as “the local response of living mammalian tissues to injury due to any agent”. It is a body defense reaction in order to eliminate or limit the spread of injurious agents, followed by the removal of the necrosed cells and tissues. Inflammatory processes are defensive or adaptive by design; however, they may contribute to distressing clinical manifestations of the disease. At the same time, they are helping to eradicate it. Inflammation is the body’s attempt at self-protection; the aim being to remove harmful stimuli, including damaged cells, irritants, or pathogens, and begin the healing process.
Inflammation does not mean infection, even when an infection causes inflammation. Infection is caused by a bacterium, virus, or fungus, while inflammation is the body’s response to it. Any injury, including an invasion by microorganisms, causes inflammation in the affected area. Inflammation, a complex reaction, results from many different conditions. The damaged tissue releases substances that cause inflammation and that direct the immune system to do the following:
- Attack and kill any invaders.
- Dispose of dead and damaged tissue.
- Begin the process of repair.
Table of Contents
Etiology of Inflammation
The agents causing inflammation may be as follows:
- Physical agents: Heat, cold, radiation, and mechanical trauma.
- Chemical agents: Organic and inorganic poisons.
- Infective agents: Bacteria, virus, and their toxins.
- Immunological agents: Cell-mediated and antigen-antibody reaction.
Cardinal signs of inflammation
The Roman writer Celsus in 1st Century A.D. named the famous four cardinal signs of inflammation:
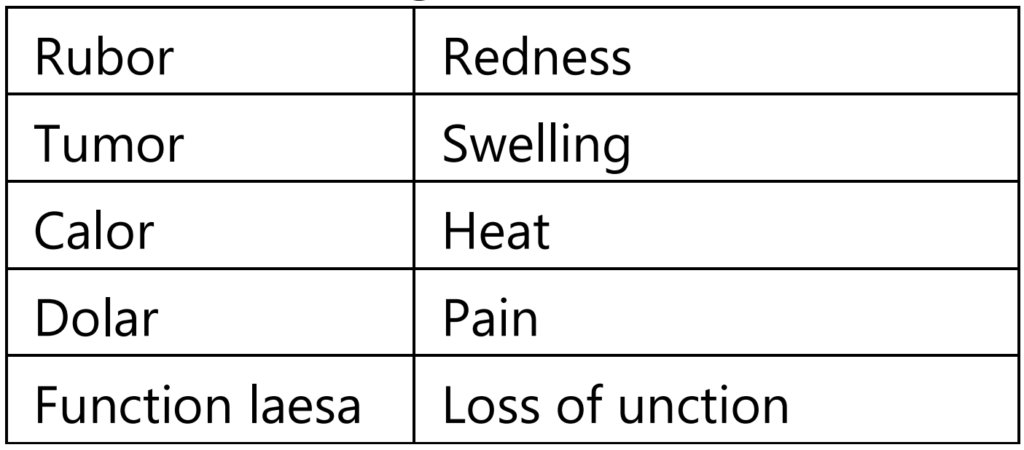
- Rubor: Latin term for “redness”. This is because the capillaries are filled up with more blood than usual.
- Tumor: a Latin term for “swelling”. Caused by an accumulation of fluid.
- Calor: Latin term for “heat”. As with the reason for the redness, more blood in the affected area makes it feel hot to the touch.
- Dolor: Latin term for “pain”. The inflamed area is likely to be painful, especially when touched. Chemicals that stimulate nerve endings are released, making the area much more sensitive.
- To these, the fifth sign function less (loss of function) was later added by Virchow.
Types of Inflammation
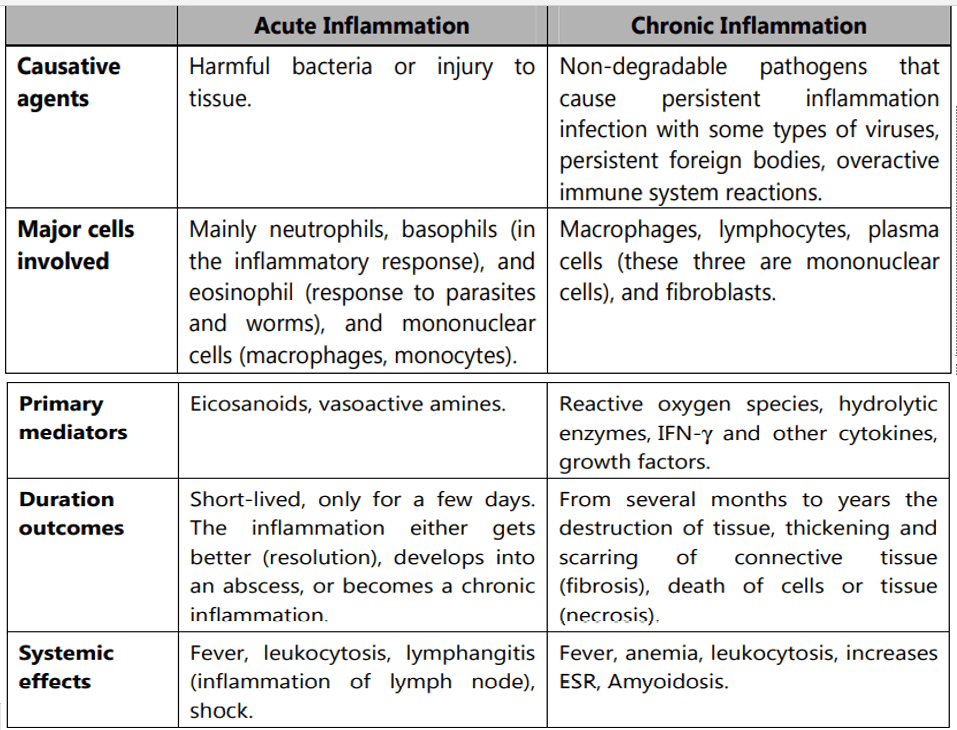
Depending upon the defense capacity of the host and duration of response, inflammation can be classified as acute or chronic.
Acute inflammation:
Acute inflammation is of short duration and represents the early body reaction and is usually followed by healing.
Examples of diseases, conditions, and situations that can result in acute inflammation include:
- Acute bronchitis
- Sore throat from a cold or flu
- A scratch/cut on the skin
- Acute appendicitis
- Acute dermatitis
- Acute tonsillitis
- Acute infective meningitis
- Acute sinusitis
Chronic inflammation:
Chronic inflammation is of a longer duration and occurs either after the causative agents of acute inflammation persists for a long time or the stimulus that induces chronic inflammation from the beginning.
The characteristic features of chronic inflammation are the presence of chronic inflammatory cells such as lymphocytes, plasma cells, and macrophages.
Macrophage (phagocytic cells) derived from a monocyte, walls of blood vessels, and loose connective tissue. They interact with lymphocytes to facilitate antibody production.
Examples of diseases and conditions with chronic inflammation include:
- Asthma
- Chronic peptic ulcer
- Tuberculosis
- Rheumatoid arthritis
- Chronic periodontitis
- Ulcerative colitis and Crohn’s disease
- Chronic sinusitis
- Chronic active hepatitis.
The basic mechanism involved in process of inflammation
Acute Inflammation
The changes in acute inflammation can be conveniently described under the following two headings.
- Vascular Events
- Hemodynamic changes.
- Altered vascular permeability.
2. Cellular Events
- Exudation of leukocytes.
- Phagocytosis.
Vascular Events:
Alteration in the microvasculature (arterioles, capillaries, and venules) is the earliest response to tissue injury. These alterations include hemodynamic changes and changes in vascular permeability.
a) Hemodynamic changes:
The earliest features of inflammatory response result from the change in the vascular flow and caliber of small blood vessels in the injured tissue.
The sequence of these changes is as under
- Irrespective of the injury, the immediate vascular response is the transient vasoconstriction of arterioles. With the mild form of injury, the blood flow may be re-established in 3-5 seconds. While with more severe injury, the vasoconstriction may last for about 5 min.
- Next follows persistent progressive vasodilatation which involves mainly the arterioles and lesser extents to venules and capillaries. This change is obvious within half an hour of injury. Vasodilatation results in increased blood volume in the microvascular bed of the area, which is responsible for redness and warmth at the site of acute inflammation.
- Progressive vasodilatation in turn may elevate the local hydrostatic pressure resulting in the transudation of fluid into the extracellular space. This is responsible for swelling at the local site of acute inflammation.
- Slowing or stasis is attributed to increased permeability of microvasculature that results in increased concentration of red cells and thus raised blood viscosity.
- Slowing or stasis is followed by leukocytes’ margination or peripheral orientation of leukocytes (mainly neutrophils) along the vascular endothelium. The leucocytes stick to the vascular endothelium briefly, and then move and migrate through the gaps between the endothelial cells into the extravascular space known as emigration.
The features of hemodynamic changes in inflammation are best demonstrated by the Lewis experiment. Lewis induced the changes in the skin of the inner aspect of the forearm by firm stroking with a blunt point. The reaction so elicited is known as a triple response or red line response consisting of the following:
- Redline appears within a few seconds following stroking and is due to local vasodilation of capillaries and venules.
- Flares are the bright reddish appearance or flush surrounding the red line and result from vasodilation of the adjacent arterioles.
- Wheal is the swelling or edema of the surrounding skin occurring due to the transudation of fluid into the extravascular space.
b) Altered vascular permeability:
In acute inflammation, the normally non-permeable endothelial layer of microvasculature becomes leaky. This is explained by one or more of the following mechanisms.
- Contraction of endothelial cells: This is the most common mechanism of increased leakiness that affects venules exclusively, while capillaries and arterioles remain unaffected. The endothelial cells develop temporary gaps between them due to their contraction resulting in vascular leakiness. It is mediated by the release of histamine, bradykinin, and other chemical mediators. The response begins immediately after injury, is usually reversible, and is for a short duration (15-30 minutes).
- Retraction of endothelial cells: In this mechanism, there is structural re-organization of the cytoskeleton of endothelial cells that causes reversible retraction at the intercellular junctions. This change affects the venules and is mediated by cytokines such as Interleukin-1 (IL-1) and tumor necrosis factor α (TNF-α). The onset of response takes 4-6 hours after injury and lasts for 2-4 hours or more.
- Direct injury to endothelial cells: Direct injury to the endothelium causes cell necrosis and the appearance of physical gaps at the sites of detached endothelial cells. The process of thrombosis is initiated at the site of damaged endothelial cells. The change affects all levels of the microvasculature. The increased permeability may either appear immediately after injury and last for several hours or days (immediately sustained leakage) or may occur after a delay of 2-12 hours and last for hours to days (delayed prolonged leakage).
- Endothelial injury mediated by leukocytes: Adherence of leukocytes to the endothelium at the site of inflammation may result in the activation of leukocytes. The activated leukocytes release proteolytic enzymes and toxic oxygen species which may cause endothelial injury and increased vascular leakiness. This form of increased vascular leakiness affects only venules is a late response.
- Other Mechanism: In addition, the newly formed capillaries during the process of repair are excessively leaky.
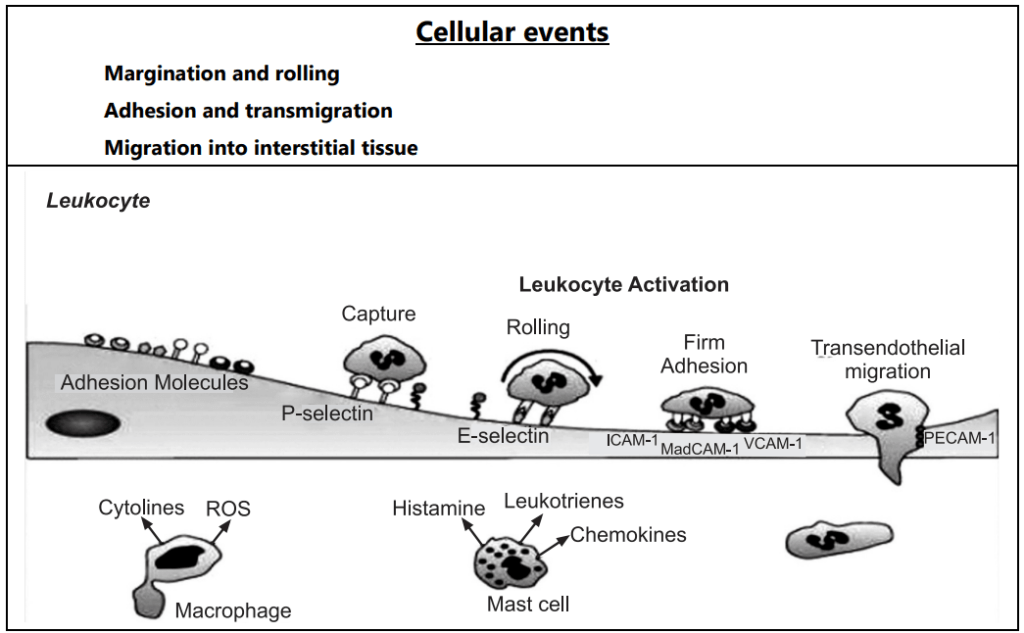
Cellular Events:
Exudation of leukocytes:
The escape of leukocytes from the lumen of the microvasculature to the interstitial tissue is the most important feature of inflammatory response. In acute inflammation, polymorphonuclear neutrophils comprise the first line of body defense, followed later by monocytes and macrophages. The changes leading to the migration of leukocytes are as follows:
- Changes in the formed elements of blood: In the early stage of inflammation the rate of flow of blood increases due to vasodilation. But subsequently, there is slowing or stasis of the blood stream. With stasis, changes in the normal axial flow of blood in the microcirculation take place. The normal axial flow consists of a central stream of cells comprised of leukocytes and RBC and a peripheral cell-free layer of plasma close to the vessel wall. Due to slowing and stasis, the central stream of cells widens and the peripheral plasma zone becomes narrower because of loss of plasma by exudation. This phenomenon is known as ‘margination’. As a result of redistribution, the neutrophils of the central column come close to the vessel wall, this is known as ‘pavementing’.
- Rolling and Adhesion: Peripherally marginated and pavemented neutrophils slowly roll over the endothelial cell lining of the vessel wall (Rolling phase). Neutrophils first roll among the surface of the endothelium in a process mediated by selectins, adhesion molecules that are expressed by endothelial cells and that bind reversibly to sites on the leukocyte membrane. Later, neutrophils become firmly adherent to the endothelium by binding of leukocyte adhesion molecules (Selectins, Integrins, Intercellular adhesion molecules-1, vascular cell adhesion molecule-1, platelet endothelial cell adhesion molecule-1, etc.) This is followed by the transient bond between leukocytes and endothelial cells becoming firmer. (Adhesion phase). The following adhesion molecules bring about rolling and adhesion phases.
- Transmigration: After sticking neutrophils to the endothelium, the former move along the endothelial cell is found where the neutrophils throw out cytoplasmic pseudopods. Subsequently, the neutrophils are lodged between the endothelial cells and cross the basement membrane by damaging it locally with secreted collagenase and escape out into the extravascular space. This is known as ‘transmigration’. The damaged basement membrane is repaired almost immediately, simultaneous to the emigration of leukocytes, escape of RBCs through the gaps between the endothelial cells. It is a passive phenomenon, RBCs being forced out either by raised hydrostatic pressure or may escape through the endothelial defects left after the emigration of leukocytes.
- Chemotaxis: The movement of leukocytes from the vessel lumen into a damaged area is called chemotaxis and is mediated by a substance known as chemotactic factors that diffuse from the area of tissue damage. All granulocytes and monocytes respond to chemotactic factors and move along a concentration gradient. Chemo attractants can be exogenous or endogenous. Most exogenous chemotactic factors are bacterial or other microbial products. There are many endogenous chemotactic factors like fibrin, fibrinopeptides, bacterial components, IL-8, IL-5, histamine, monocyte chemotactic factors (MCP-1 & MCP-5).
Chemotactic factors bind to receptors on the surface of leukocytes and activate secondary messenger systems. These messenger systems work by stimulating increased intracytoplasmic calcium. The calcium then interacts with the cytoskeleton resulting in active movement of the cell.
Phagocytosis
It is defined as “the process of engulfment of solid particulate material by the cells”.
There are two main types of phagocytic cells:
- Polymorphonuclear neutrophils (PMNs) appear early in acute inflammatory response also called macrophages.
- Circulating Monocytes and fixed tissue mononuclear phagocytes are called macrophages.
Neutrophils and macrophages on reaching the tissue space produce several proteolytic enzymes lysozyme, protease, collagenase, elastase, lipase, proteinase, gelatinase, and acid hydrolases. These enzymes degrade collagen and the extracellular matrix that will kill bacteria.
Make sure you also check our other amazing Article on: Gonorrhea