Total quality management (TQM) is a term that is widely used when talking about how good or poor a product or service is. However, defining this term is not so easy. In most cases, it refers to how well the product can match the specifications and meet the customer’s expectations. ISO defines quality as “The degree to which a set of inherent characteristics fulfills the requirements.”
Table of Contents
Evolution of Quality management
In the early 1900s, businesses were looking for ways to reduce the number of faulty products being produced. In 1911, Frederick W. Taylor published a book titled “The Principles of Scientific Management” which focused on how organizations must use people effectively. He proposed the concept of ‘clearly defined tasks’ and ‘standard conditions’ and proposed people be assigned for the purpose of ‘inspection’ of goods. This led to the birth of the “Inspection Department” and its focus was on preventing defects which in turn, developed into the concept of Quality Control.
During 1930s, Dr. W. Shewhart developed methods for statistical analysis of production processes. Through the use of a ‘control chart,’ he showed how variations in manufacturing processes lead to variations in the quality of the product. His theory of Statistical Quality Control highlighted the detection and control of problems in quality by testing several samples at different stages of the production process.
During the 1940s, Japanese industry leaders wanted to change the quality of their products which were largely seen as cheap imitations. At their invitation, western quality experts such as W. Edwards Deming, Joseph M. Juran, and Armand V. Feigenbaum visited Japan to guide these industries with their quality needs.
Deming taught the Japanese the concepts of statistical analysis and used them for quality control. Juran’s teachings focused on the idea of getting managers involved with the quality process. In the early 60s, the Japanese firms came up with ‘quality circles’ that comprised a voluntary group of workers who met to discuss improving some aspects of their work and then presented their thoughts to the management. This led to employees feeling more motivated to contribute to the workplace. Gradually, the discussions began to center around improvements that could be made in all organizational areas and not merely the quality of products. This is how the idea of ‘Total Quality’ originated.
In 1969, Feigenbaum presented a paper at the first international conference on quality control in Tokyo. In it, he used the term ‘Total Quality’ for the first time. Around the same time, Ishikawa in Japan also came up with the concept of ‘Total Quality Management (TQM)’ which differed from the western theory of total quality.
By observing the great strides made by Japan in dealing with quality issues, industry leaders in the West began their own set of quality initiatives in the 1980s and 1990s. These ideas covered a large spectrum of techniques and strategies, and it was all covered by the term ‘Total Quality Management or “TQM.”
Definition of Total quality management (TQM)
Today, TQM has evolved greatly from those initial days, and every day, there is some other innovation being added to the term. TQM is defined in several ways, such as:
‘TQM is a management system and philosophy that strives towards constant organizational improvement in order to achieve excellence and ensure customer satisfaction and loyalty.’
‘TQM is the continued process of detecting/reducing/eliminating errors in manufacturing,
streamlining the process of supply chain management, improving customer experience, and
ensuring employees are well-trained.’
‘TQM is a structured approach to organizational management with a process focused on improving the quality of outputs of an organization, including services and goods, by the constant improvement of its internal practices.’
‘TQM is an organizational management philosophy seeking to continuously improve the quality of processes and products.’
Using a set of management and quality tools, the TQM approach seeks to increase business even as it reduces loss due to improper practices. Being a highly adaptable concept, it has been widely applied in several industries in the production and service sectors.
The main components of Total Quality Management (TQM) include:
- Focus on consumer
- Analysis of process
- Work in quality teams
- Systematic analysis of problems
- Implement planned changes and evaluate results
- Use data to identify problems and solutions
- Implement changes
TQM focuses on continuous improvement at all levels right from planning up to execution on the shop floor. The main concern is to avoid mistakes and thus, prevent defects in the products. By regular improvement of personnel, equipment, processes, and capabilities, seeks to ensure quality that is consistent. TQM is also based on the main principle that mistakes are often a result of faulty processes and systems and not of individuals per se. By identifying the causes of such mistakes, it is possible to eliminate them through three mechanisms:
- Prevent errors from occurring.
- Where prevention is not possible, early detection to prevent the mistake causing damage down the chain.
- Immediate correction of process if mistakes recur.
Key Element of TQM
There are eight key elements on which an organization must focus to implement TQM with success:
- Ethics
- Integrity
- Trust
- Training
- Teamwork
- Leadership
- Recognition
- Communication
These eight elements are further clubbed into four groups as follows, based on their function:
- Group I – Foundation – Ethics, Integrity, and Trust
- Group II – Building Bricks – Training, Teamwork, and Leadership
- Group III – Binding Mortar – Communication
- Group IV – Roof – Recognition
Foundation
A foundation of ethics, integrity, and trust helps to create an open and fair environment that fosters involvement by everyone in the organization. Ethics deals with what is good and bad in a given situation, both at the individual and the organizational levels. Integrity refers to the honesty with which one adheres to facts. When ethics are followed with integrity, it leads to the development of trust, the third element of the ‘foundation’, which creates an environment of cooperativeness.
Building Bricks
Employees need to be trained in performing their duties right and in problem-solving. They must also be trained in interacting with others, to do their job better through teamwork. Any team is only as good as its leader, though, and so, there has to be inspirational leadership by someone who understands TQM and is committed to it in daily practice.
Binding Mortar
The link which binds all elements of TQM is communication which refers to a common understanding of the message by both the sender and receiver. Openness in communication between members of an organization, and with vendors, and customers is key to the success of TQM.
Roof
Recognizing the contributions of people in an organization, whether for teams or individuals, is the final element in TQM. When employees receive recognition, it brings about a leap in their self-esteem and such employees are more motivated which ultimately leads to better productivity and quality of work they do.
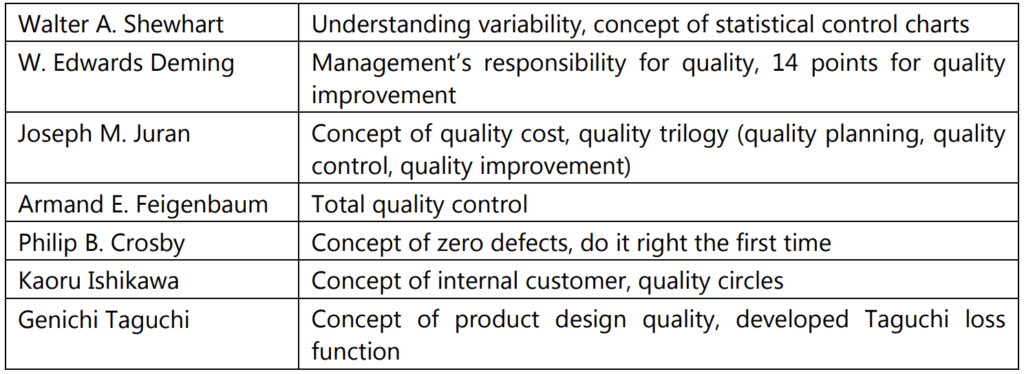
Philosophies of TQM
Several quality experts have given their viewpoints on how to achieve quality. Some of the most important concepts have been summed up in the table below:
Advantages of Total quality management
- Innovation in processes
- Greater productivity
- Reduced defects in product
- Increased customer satisfaction
- Higher profitability and reduced costs
- Higher employee morale
- Better adaptability to changing market conditions
- Increased competitiveness
TQM in the drug industry
Pharmaceutical literature does not offer any common definition for the term ‘quality’. The idea implied in most definitions is the idea that the drug product has to meet customer needs. Customers cannot assess the quality of their drugs and this is the reason why governments have to step in with rules and regulations to ensure the quality of medicines.
A study of the history of drug regulations reveals that most laws were enacted in response to tragedies that occurred when patients received drugs that were contaminated or not properly processed or incorrectly labeled. This led to a focus by drug makers on Quality Control – the process of testing the quality of products manufactured in their facilities.
As time evolved, and pharmaceutical processes grew more complex, it gradually came to be realized that no process is perfect all the time, and there are drifts from the normal functioning. The best testing methods may miss detecting a problem because the destructive nature of the testing means only a few samples drawn at random can be tested. Thus, there was a realization that relying on mere testing of products at the end of the manufacturing process is an ineffective (and costly) way of trying to ensure quality.
According to regulatory bodies across the world, high-quality drug products are those that can be relied upon to deliver the desired clinical effects with consistency. Consumers of medicines need to know for sure that the drug product they are consuming is of good quality, safe for consumption, and will be effective in relieving them of their ailment. This quality can be guaranteed only if the products have it built into them right from the very first stage of the design of the product and process. Realization of this basic principle led to the International Conference on Harmonization (ICH) in 2002, introducing a hitherto unheard-of term in the drug processing field – the concept of ‘Quality by Design’ or QbD.
Quality by Design
The ICH guideline Q8 (R2) Pharmaceutical Development defines QbD as, “A systematic approach to development that begins with predefined objectives, emphasizes product, process understanding, and process control, based on sound science and quality risk management.”
To achieve the objective of QbD, it is important to understand product characteristics and study process characteristics using a combination of prior knowledge and experimental studies. From this data generated during product development, it becomes possible to decide which quality parameters in starting materials are the most important, and which critical processing factors need to be controlled to achieve a product with all the desired quality attributes.
The ICH Q10 guideline says that a Pharmaceutical Quality System is one that “assures that the desired product quality is routinely met, suitable process performance is achieved, the set of controls are appropriate, improvement opportunities are identified and evaluated, and the body of knowledge is continually expanded.”
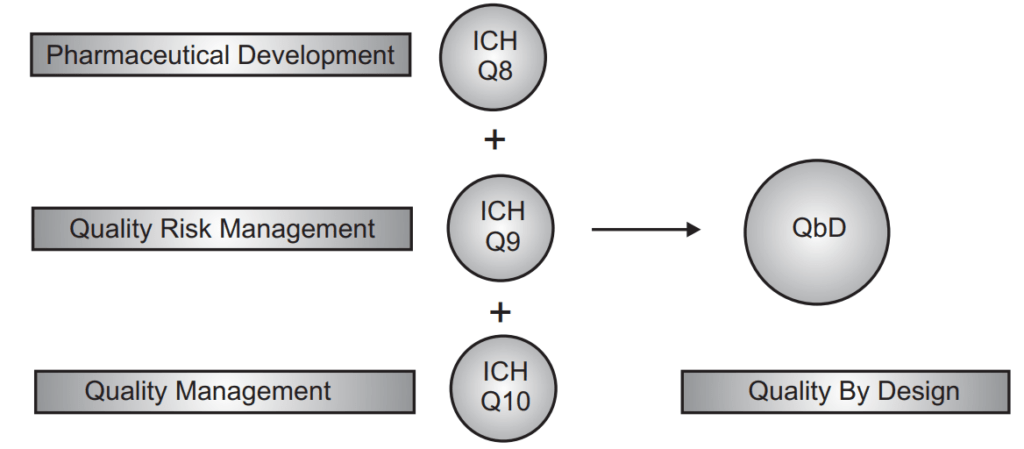
In other words, a robust pharmaceutical quality system is the key to supplying customers with high-quality drugs. Such a system has the following characteristics:
- Aligned with requirements of current Good Manufacturing Practices (cGMP)
- Science-based and risk-based
- Comprehensive
- Proactive and accountable
Developing such pharmaceutical quality systems is the key to meeting patient requirements efficiently. Because they deliver consistent quality, these systems also serve to increase the confidence of regulatory bodies about the organization’s commitment to quality.
Make sure you also check our other amazing Article on: Schedule M
