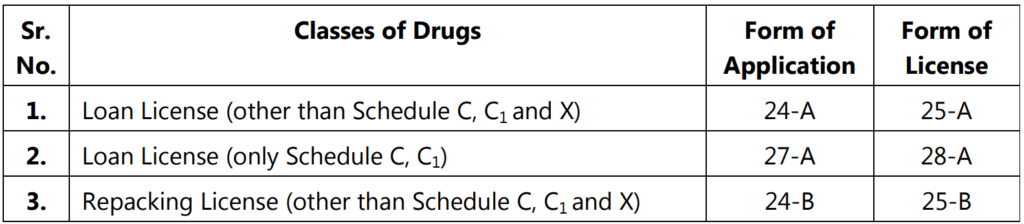
A loan license is issued by the licensing authority, to a person who does not own, arrangements for the manufacturer but intends to avail the manufacturing facilities owned by another licensee.
- In the case of pharmacy business is operating its business in more than two states then it is required to obtain a drug license in each state where the business is carried on. A separate license shall be issued in case drugs are sold in more than one place.
- After the license is granted to the business, the licensee must ensure that all the conditions of the drug license must be complied with during business. In the case of any changes or modifications in business activity authority must be informed and all the registers, records, and forms must be maintained in a specified manner.
- The licensing authority shall, before the grant of a loan license, satisfy himself that the manufacturing unit has adequate equipment, staff, capacity for manufacture, and facilities for testing, to undertake the manufacture on behalf of the applicant for a loan license.
- Application for the manufacture of more than ten items for each category of the drug on a loan license shall be accompanied by an additional fee of rupees three hundred per additional item specified in Schedule M and Schedule M3.
- The licensee shall allow Inspector to inspect the premises and satisfy himself that only examination is conducted.
- The licensee shall maintain the ‘Inspection Book’ in Form 35.
- The licensee shall comply with further requirements specified by the authority.
- The licensee shall test each batch of raw materials used and each batch of the final product and also maintain the records of manufacture and testing of each batch as per schedule U.
- Shall maintain the reference samples from each batch for the period of 3 years.
- Report to the licensing authority, regarding the changes in expert staff or change in the manufacture or testing units.
- For Schedule C and C1 drugs, the licensee shall furnish the data of stability and date of expiry to the licensing authority.
- If the licensing authority is satisfied that a loan license is defaced damaged or lost or otherwise rendered useless, he may, on payment of a fee of rupees one thousand, issue a duplicate license.
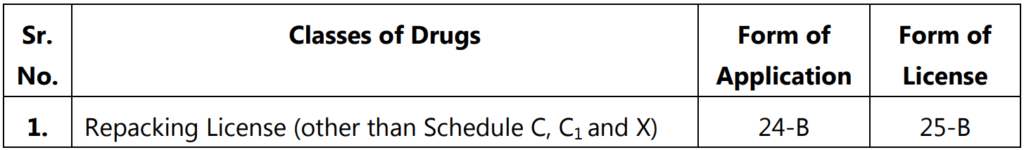
Repacking License
It is granted for the purpose of breaking up any drug than those specified in Schedule C and C1.
- Repacking of drugs should be conducted under hygienic conditions under the personal supervision of a competent person, approved by the licensing authority.
- The licensee must provide and maintain adequate arrangements for carrying out tests of drugs repacked, in the specified place by the authority.
- The licensee shall allow Inspector to inspect the premises and to take samples of repacked drugs.
- The licensee shall test each batch of raw materials used and each batch of the final product and also maintain the records of manufacture and testing of each batch as per schedule U. Records must be retained for 5 years from the date of repacking.
- Licensee must allow the Inspector to inspect all the registers and records maintained.
- The licensee shall maintain the ‘Inspection Book’ in Form 35.
- Shall maintain the reference samples from each batch of repacked drugs, for the specified period.
- Licenses remain valid for a period of 5 years from the date its granted or renewed unless suspended or canceled.
Make sure you also check our other amazing Article on: Manufacture of Drugs
