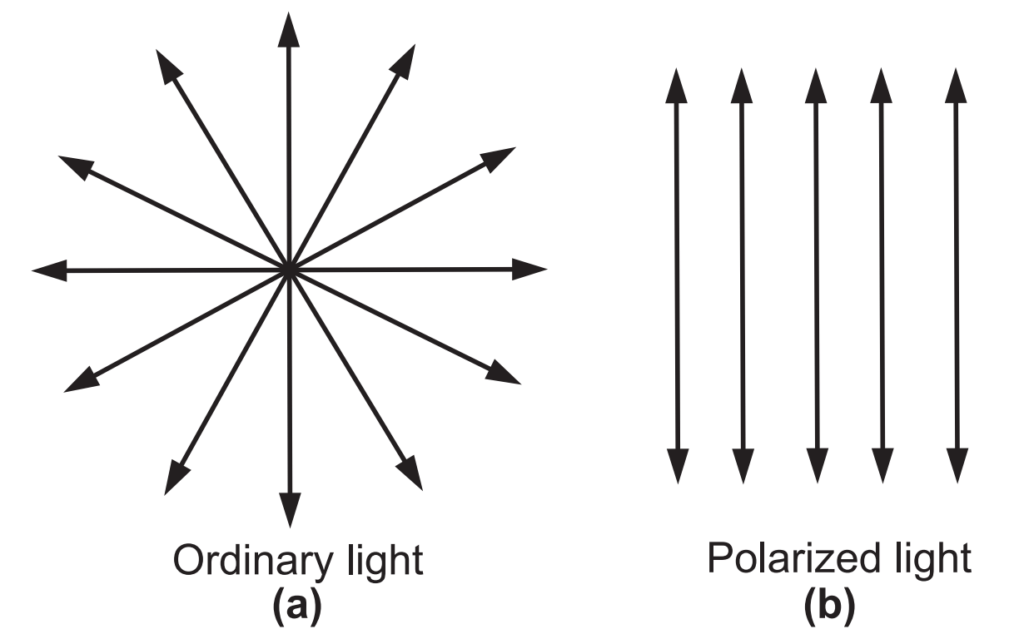
Ordinary light consists of vibrations, which are evenly distributed in all directions in a plane perpendicular to the direction of propagation, called as unpolarised light, Fig. 1.1 (a). When the vibrations of light are restricted to only one plane, the light is said to be polarized light, Fig. 1.1 (b). Some substances that rotate the plane of polarized light are called as optically active substances. Optical rotation is the property of an optically active substance is measured as the angle of rotation. The property in which rotation of plane-polarized light is observed is known as optical activity. Optically active substances include organic molecules with a central carbon atom to which four different groups are attached, making the molecule very asymmetric (chiral carbon).
Similarly, laevulose, more commonly known as fructose causes the plane of polarization to rotate to the left. Fructose is even more strongly Levorotatory than the glucose. The substance which rotates the plane of polarized light to the right or clockwise when viewed in the direction of light propagation is called dextrorotatory (d) or (+) substance.
The use of one name for glucose, dextrose, refers to the fact that it causes linearly polarized light to rotate to the right or dexter side. Those optically active substances that rotate plane-polarized light to the left or counterclockwise are known as levorotatory (l) or (−) substances. Laevulose, more commonly known as fructose causes the plane of polarization to the left. Fructose is even more strongly Levorotatory than the glucose. Other examples of optically active substances are lactic acid, tartaric acid, 2-methyl -1-butanol, etc. The optical rotation occurs because of optically active substances have different refractive indices for left and right polarized light. Another way to make this statement is that left and right polarized light travel through an optically active substance at different velocities. Optical activity is considered to be due to the interaction of plane-polarized radiations with electrons in molecules which shows electronic polarization. This interaction rotates the direction of vibration of radiation by altering the electric field.
Optically active substances can be categorized into two types:
- Those which are optically active only in the crystal state due to their characteristic crystal structure and becomes optically inactive in the fused or dissolved state, for example, sodium chlorate, quartz crystal, etc and,
- Those which show optical activity in all states viz. Crystalline (solid), fused (liquid), and gaseous state, due to their structural configurations.
Table of Contents
Specific Rotation
When polarized light passes through an optically active substance, all the molecules in the path of light rotate the plane of polarization by some constant amount which is a characteristic of that substance. The total rotation in the emergent light beam is proportional to the path length (l) and density (ρ) of the substance. This relation is mathematically expressed as
θ ∝ ρ … (1)
∴ θ = [α] ρ…(2)
where, α, is proportionality constant called as specific rotation. The amount of optical rotation depends on the number of optically active species through which the light passes and thus depends on both the sample path length and analyte concentration. Specific rotation provides a normalized quantity to correct for this dependence, and is defined as;
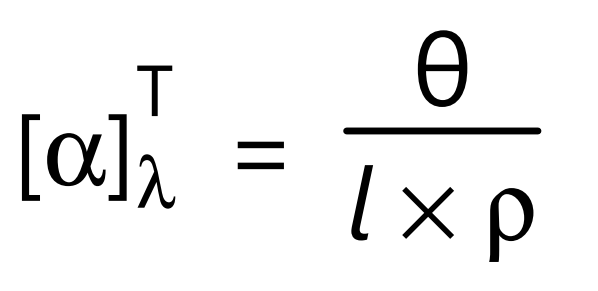
where, θ, is measured optical angle of rotation in deg cm2 /g or degrees, l is sample path length in decimeters (dm) and ρ is density if the substance is pure liquid, λ is the wavelength of light used for observation, usually 589 nm, the D line of a sodium lamp unless otherwise specified and T is the temperature in °C.
The angle of rotation changes with a change in the wavelength of light used. Therefore the specific rotation also changes with the wavelength of light used. The graph of specific rotation versus wavelength shows an inflection and then passes through zero at the wavelength of maximum absorption of polarized light. This change in specific rotation is called as a cotton effect. Substances that show maximum rotation before passing through zero due to a smaller wavelength of polarized light are said to show a positive cotton effect. If specific rotation shows maximum value after passing through zero, the substance shows a negative cotton effect. Cotton effects are helpful in characterizing enantiomers, especially in the structural elucidation of organic compounds. The variation in the angle of rotation with a wavelength of light is called the optical rotatory dispersion. It is recorded using a spectropolarimeter, which has a tungsten lamp and a canning monochromator as a light source. A motorized mount rotates the analyzer to maintain a minimum signal at the detector. Usually, a modulation is introduced into the polarization angle of the light beam so that DC signals to the analyzer motor.
Molar Rotation
Molar rotation is a characteristic property of optically active substances. It is obtained from the multiplication of specific rotation and molecular weight of the compound as
µ = M[α] × 100…(3)
where µ is molar rotation, M is molecular weight, and [α] is specific rotation.
Enantiomeric Purity
The molecules that are non-superimposable mirror images are called enantiomers. In the case of optically active substances if only one enantiomer is present then the substance is considered to be optically pure, while if it consists of mixtures of two enantiomers (a racemic mixture), it will not rotate the plane of polarized light and is optically inactive. A mixture that contains one enantiomer in excess displays a net plane of polarization which is characteristic of the enantiomer that is in excess. The optical purity or the enantiomeric excess (%ee) of a sample can be determined as follows:
Optical purity = % enantiomeric excess
= % enantiomer1 − % enantiomer2
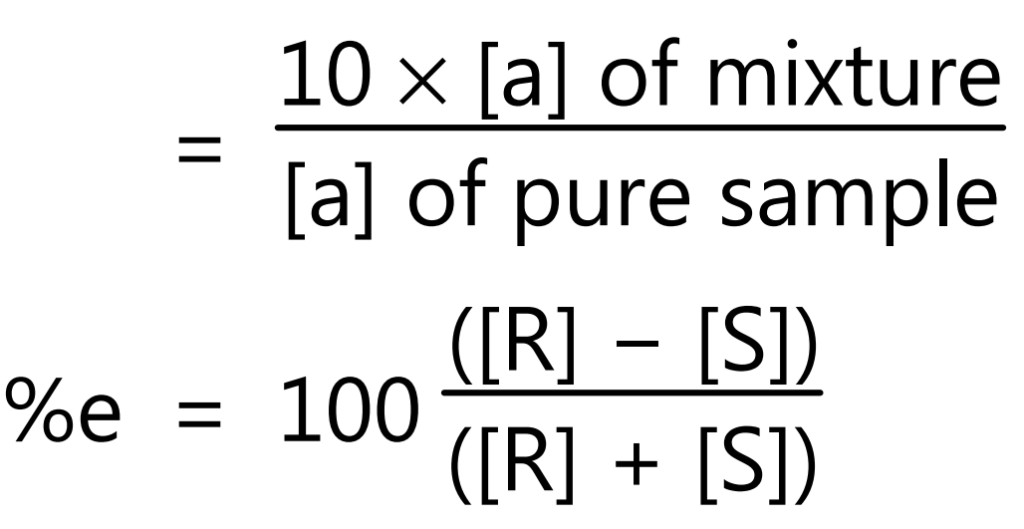
where, [R] and [S] are the concentrations of R and S isomers, respectively.
Measurement of Optical Activity
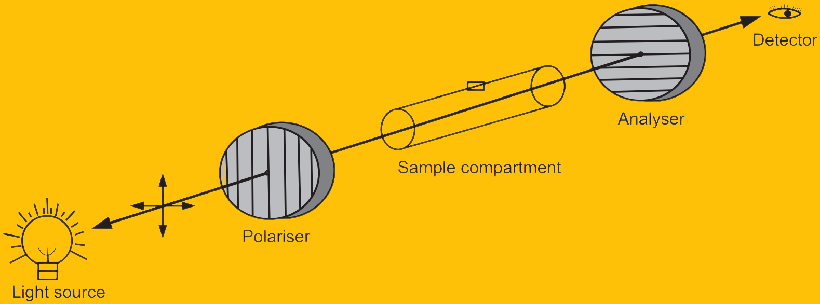
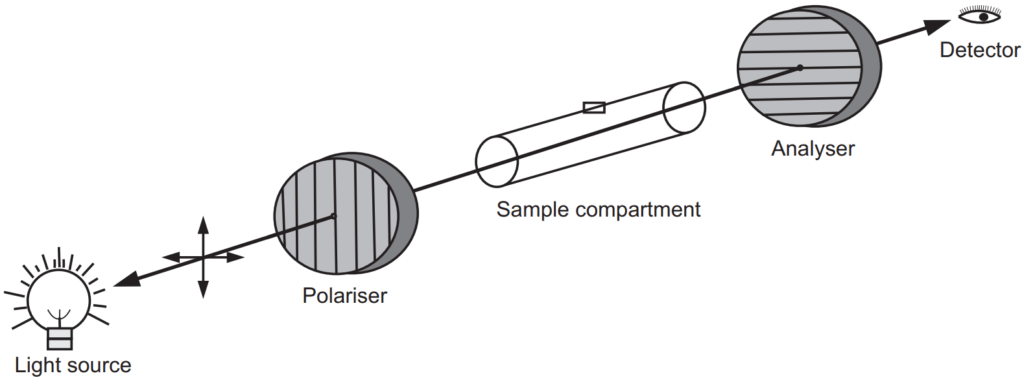
Measurement of the orientation of plane-polarized light is called polarimetry, and the instrument used is called a polarimeter. The simplest polarimeter, Fig. 1.2 , consists of the monochromatic light source, a polarizer, a sample cell, a second polarizer which is called the analyzer, and a light detector. Polariser and analyzer are made up of Nicol prisms. When the analyzer is oriented 90° to the polarizer no light reaches to the detector. The polarizer is placed near to the light source while the analyzer is placed between the sample cell and the detector. The sample cell of suitable size and capacity with outward projection at the center, to trap the air bubble is usually used. When an optically active substance is placed in the sample cell and a beam of light is passed through, it rotates the polarization of the light reaching the analyzer so that there is a component that reaches the detector. The angle that the analyzer must be rotated from the original position is the optical rotation.
For a pure substance in solution, if the color and the path length are fixed and the specific rotation is known, then observed rotation can be used to calculate the concentration. Optical activity is useful in studying the structure of anisotropic materials, and for checking the purity and identifying chiral mixtures. Adulterations in the optically active substances can be determined from the optical rotation. For example, the optical rotation of honey is opposite to that of sugar due to the presence of fructose and glucose and hence can be determined from the optical rotation. Chemical kinetic studies are also carried out by determining concentration at different time intervals as in the case of sugar inversion. Polarimetry is used in the analysis of various drugs and pharmaceutical formulations such as Adrenaline Bitartrate, anticoagulant Citrate Dextrose Solution, Dextran 40 Injection, Dextrose Injection, Sodium Chloride, and Dextrose injection, etc.
Make sure you also check our other amazing Article on : Dipole Moment