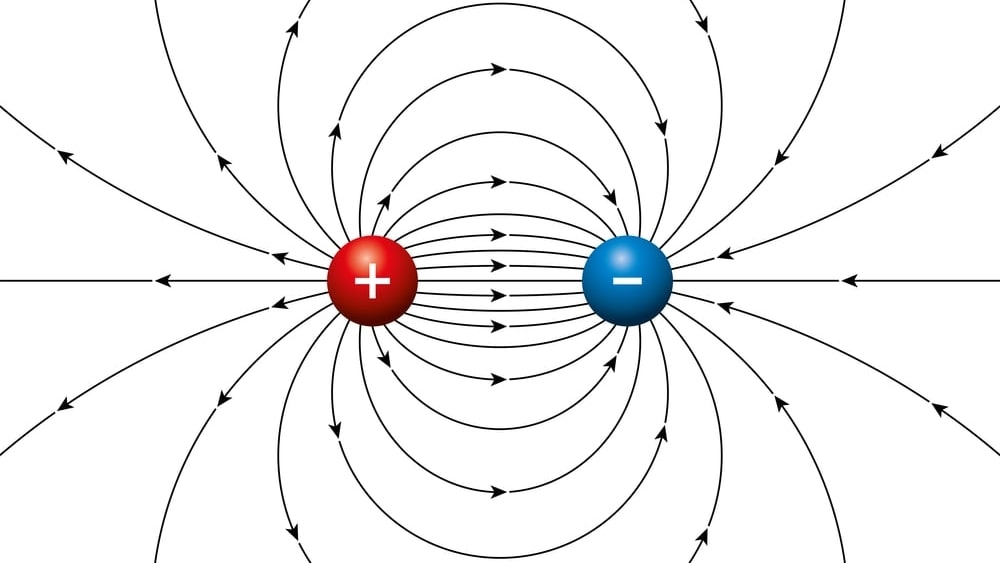
A dipole is a pair of separated opposite electric charges. An electric dipole is an assemblage of atoms or subatomic particles having equal electric charges of opposite signs separated by a finite distance. Dipoles are characterized by their dipole moment, a vector quantity with a magnitude equal to the product of charge or magnetic strength of one of the poles, and the distance separating the two poles.

µ = q × r
where µ is dipole moment, q is a charge on atom and r is a distance of separation of charge.
The direction of the dipole moment corresponds for electric dipoles, to the direction from the negative to the positive charge. The direction of an electric field is defined as the direction of the force on a positive charge, the electric field lines away from a positive charge and toward a negative charge.
Table of Contents
Molecular Dipoles:
Many molecules have dipole moments due to non-uniform distributions of positive and negative charges on their various atoms. In the case of HCl, the bonding electron pair is not shared equally rather is attracted towards the more electronegative chlorine atom due to its higher electro-negativity which pulls the electrons towards it. It leads to the development of a positive charge to the H atom and a negative charge to the chlorine atom.
A molecule having positive and negative charges at either terminal is referred to as electric dipoles or just dipoles. Dipole moments are often stated in Debyes; The SI unit is the coulomb meter.
Molecular dipoles are of three types
Permanent dipoles: These occur when two atoms in a molecule have substantially different electro-negativity with one atom attracting electrons more than another becoming more electronegative, while the other atom becomes more electropositive.
Instantaneous dipoles: These occur due to chance when electrons happen to be more concentrated in one place than another in a molecule, creating a temporary dipole.
Induced dipole: These occur when one molecule with a permanent dipole repels another molecule’s electrons, inducing a dipole moment in that molecule.
In a diatomic molecule, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom. In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4), there is no overall dipole moment though the individual C-F bonds are polar.
Molecular dipole moments:
In most molecules even though the total charge is zero, the nature of the chemical bond is such that the positive and negative charges do not overlap. These molecules are said to be polar because they possess a permanent dipole moment. An example of this type is the water molecule. The molecules with mirror symmetry like oxygen, nitrogen carbon dioxide, and carbon tetrachloride have no permanent dipole moments. Even if there is no permanent dipole moment, it is possible to induce a dipole moment by the application of an external electric field and is called as polarization. The magnitude of the dipole moment induced in the molecules is a measure of the polarizability of that molecular species.
Permanent dipole moment:
The permanent dipole moment differs from induced polarization in the sense that it is a permanent separation and it happens only to be in the polar but not in the non-polar molecules. These charges that separate balance out each other and therefore have a net charge of zero. The water is an example of a permanent dipole moment. The permanent dipole moment is defined as the vector sum of the individual charge moments within the molecules.
Applications of dipole moment
The structure of the molecule can be confirmed from the dipole moment values, for example, chlorobenzene, benzene, carbon dioxide, etc. The cis and trans isomers can be differentiated from dipole moment values, for example, cis and trans dichloroethylene. Dipole moments can be used to determine the percent ionic character of the bond of the molecule, e.g. H-Cl a covalent bond, the ionic characteristic is 17%. Permanent dipole moments can be correlated with the biological activities to obtain information about the physical parameters of molecules. The more soluble the molecule the easier it passes the lipoidal membrane of insects and attacks the insect’s nervous system. Therefore, the lower is the dipole moment the greater is the insecticidal action. For example, p, m, and o isomers of DDT show different insecticidal activities due to their differences in permanent dipole moment as p- isomer shows µ=1.1 and has predominant toxicity, o-isomer shows µ = 1.5 with intermediate toxicity while m-isomer shows µ = 1.9 with least toxicity. The variations in activities of different isomers are due to the greater solubilities in non-polar solvents.
Make sure you also check our other amazing Article on : Critical Solution Temperature