Pharmacognosy of Cinnamon consists of dried bark, freed from the outer cork and from the underlying parenchyma, from the shoots growing on the cut stumps of Cinnamomum zeylanicum Nees.
Synonyms: Dalchini, Ceylon Cinnamon, Cinnamon bark.
Biological source: Cinnamon consists of dried bark, freed from the outer cork and from the underlying parenchyma, from the shoots growing on the cut stumps of Cinnamomum zeylanicum Nees.
Family: Lauraceae.
Geographical source: Srilanka, Malabar Coast of India, Jamaica, and Brazil.
Cultivation and Collection of Cinnamon
It is generally cultivated by seed propagation method but sometimes plant cuttings are also preferred. It mainly needs sandy or siliceous soil which should be rich in humus. The other requirements for its better cultivation are altitude (800 to 1000 meters) and annual rainfall (200 to 250 cm). It is shade loving plant. The seeds are propagated in nursery beds in the month of June and July. The distance should be 10 cm between two plants. The plants should be watered from time to time. Generally, the seeds germinated within 20 days. Shading is provided to the plants and allowed to grow for about 1 year. Then transplantation in the open field should be done in the month of October or November or in the rainy season. The distance should be kept at least 2 meters between two plants. Weeding should be done 2 to 4 times in a year. The plants should be manured in the first year and subsequently increased depending upon the age of the plant. The fertilizers are applied first in the monsoon and second in October-November. It will encourage the growth of shoots. Coppicing should be done to induce the formation of shoots. Harvesting should be done in the rainy season because in this season peeling of bark from shoots is easy. The peeled strips are made into bundles, wrapped in coir mats, and allow to ferment for 24 hours. This will loosen the outer cork and cortex which should be removed from the curved brass knife. The collected bark contracts and is converted into quill form after drying. The smaller quills are placed in between larger quills and form compound quills. The soft and fresh quills are rolled by hand and lightly pressed so it will avoid the splitting of bark into pieces. Then the drug should be shade dried and dried quills are packed into bundles of different grades and marketed. The small pieces and debris are used for the production of Cinnamon oil. The average yield of bark is about 200- 300 kg per hectare and 2-3 kg of leaves per hectare, annually.

Description:
Cinnamon Bark:
Colour: Externally dull yellowish brown, internally dark yellowish brown
Odor: Aromatic
Taste: Warm and very refined (Sweetish and aromatic followed by a warm sensation)
Fracture: Splintery Size: Length is about 1 meter, diameter is nearly 1 cm and thickness is approximately 0.5 mm.
Shape: Compound quill form.
The wavy longitudinal striations are present on the external and internal surfaces of bark (bark freed from cork).
Cinnamon Oil:
Colour: Yellow to reddish in color.
Specific gravity: 1.00 to 1.030.
Optical rotation: 0 to − 2.
Refractive index: 1.562 to 1.582.
Chemical Constituents of Cinnamon bark
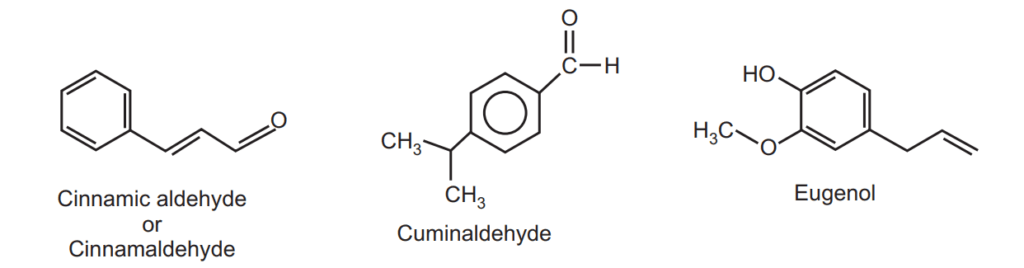
Cinnamon bark contains polycyclic diterpenes and proanthocyanidinoid oligomers. It contains volatile oils (0.5 to 1 percent), phlobatannins (1.2 percent), mucilage, calcium oxalate, starch, and mannitol (responsible for sweetish taste). The cinnamon oil obtained from the distillation method is light yellow in color and upon storage changes to reddish in color. The essential oil (5 to 20 ml/kg) is composed of phenylpropane derivatives. Cinnamon oil mainly contains cinnamaldehyde (60 to 70 percent), eugenol (5 to 10 percent), benzaldehyde, cumin aldehyde, and other terpenes such as phellandrene, pinene, cymene, caryophyllene.
Chemical Test
To a drop of volatile oil add a drop of ferric chloride solution, and a pale green color develops (cinnamaldehyde produces brown color and eugenol gives blue color which results in the formation of pale green color).
Uses:
The drug is used as an aromatic stimulant, antibacterial, antifungal, antiseptic, carminative, stomachic, and astringent. Commercially, it is also used as a spice, condiment, candy preparation, dentrifices, and perfumery. Cinnamon oil is used in urinary infection and food technology.
Cinnamon oil and cinnamaldehyde are irritating to skin and mucous membranes. They cause allergic reactions like urticaria or edema of the face and lips.
Table 1.1: Substituent and Adulterants of Cinnamon
| Sr. No | Adulterant | Characters |
| Java Cinnamon | Obtained from Cinnamomum burmanii, Family- Lauraceae, less aromatic, peeled, occurs in double quill form, contains cinnamaldehyde (74 percent) | |
| Saigon Cinnamon | Obtained from Cinnamomum burmanii, Family- Lauraceae, Bark greyish brown in color, sweet taste, contain volatile oil (2.5 percent) | |
| Cinnamon chips | Pieces of unpeeled and untrimmed bark, contain cork cells, poor yield to alcohol (90 percent) | |
| Jungle Cinnamon | Obtained from wild-grown trees, darker in color, less aromatic, slightly bitter |
Make sure you also check our other amazing Article on: Pharmacognosy of Clove
