Preparation of Amines: Reduction, replacement, or rearrangement reactions may be used to get aliphatic amines.
(A) Aliphatic amines are prepared by the reaction between either alcohol or an alkyl halide and ammonia.
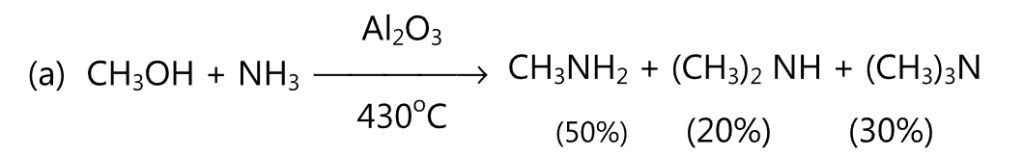
Copper chromite or alumina (Al2O3) may be used as a catalyst. Using a large excess of ammonia, the yield of primary amine may be increased.
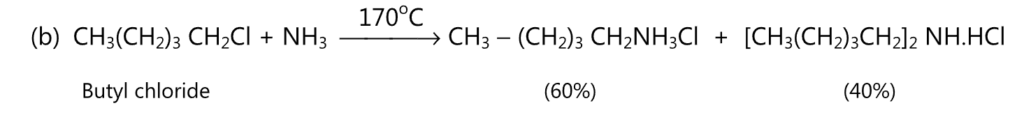
The order of reactivity of alkyl halide is
alkyl iodide > alkyl bromide > alkyl chloride
(B) Similarly by the reduction of a nitroalkane, an amine is obtained. Metal and acid or catalytic reduction may be used for this purpose.
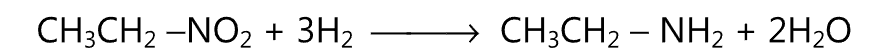
It is a commonly used method for the preparation of primary amines. One of the commonly used rearrangement reactions to get amine is Hofmann degradation or Hafmann rearrangement.
(C) Hofmann degradation may be represented by the following reaction.
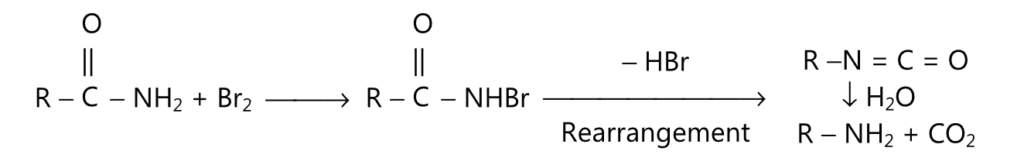
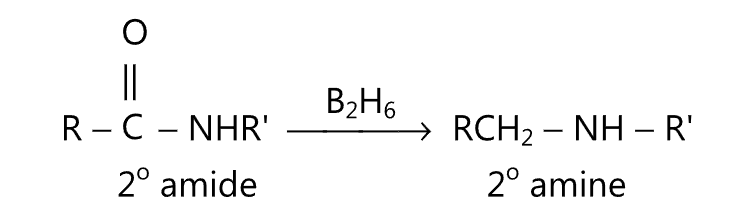
(D) Primary, secondary or tertiary amides may be reduced to corresponding amines by refluxing with diborane in tetrahydrofuran.
(E) Aldehyde or ketone may be converted to the corresponding amine by treatment with an excess of ammonia and hydrogen under pressure over Raney nickel at 60° – 150°C.
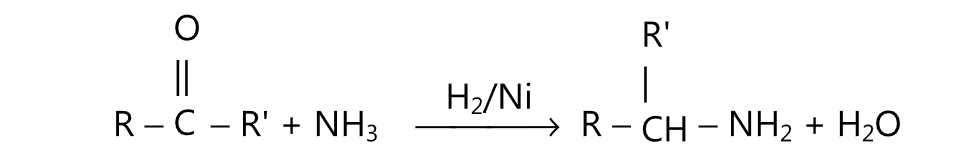
Alternatively, excess ammonium chloride and H2 over platinum catalyst may be used. Such a type of reaction is known as reductive alkylation.
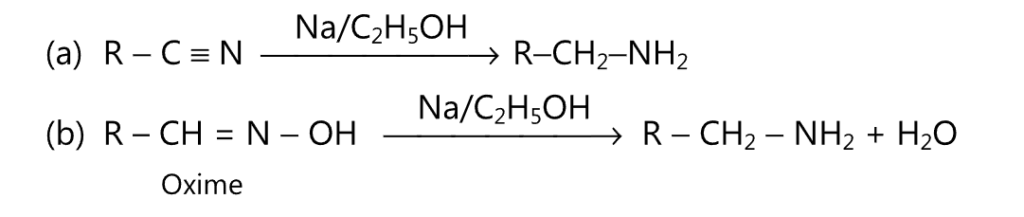
(F) Primary amines may be obtained by reduction of alkyl cyanides or oximes.
(G) Miscellaneous methods of preparation of amines :
(i) Curtius reactions :
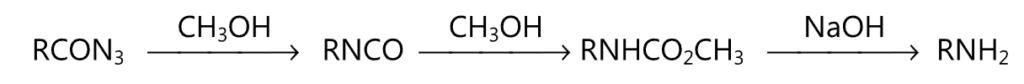
(ii) Lossen rearrangement:
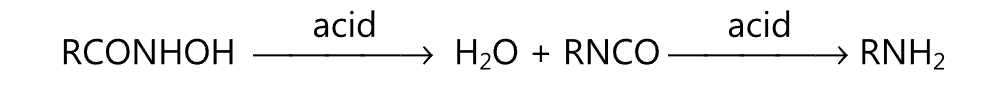
(iii) Wurtz reaction:
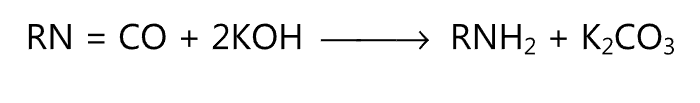
(iv) Leuckart reaction:
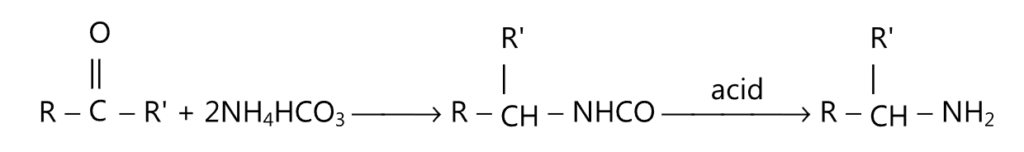
(v) Schmidt reaction:
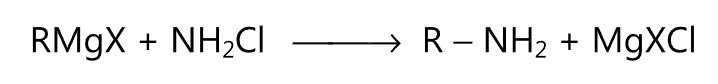
(vi) Grignard reaction:
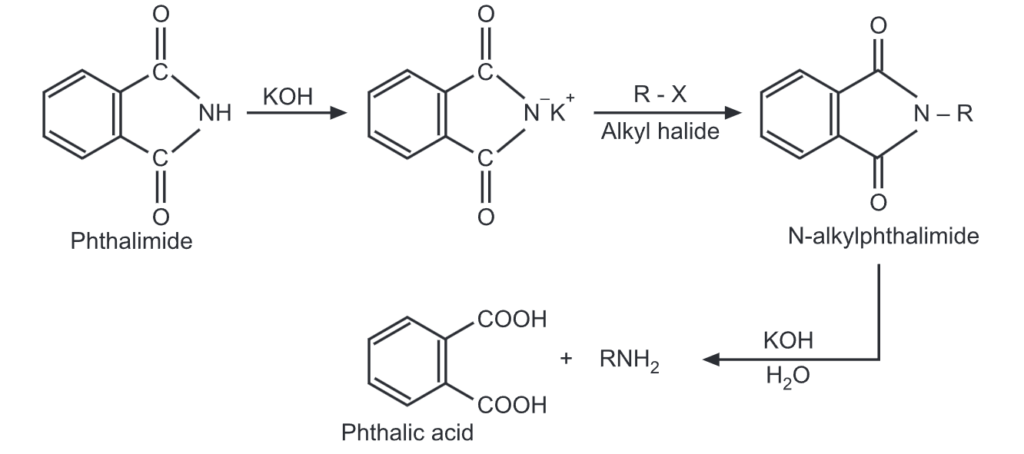
(vii) Gabriel’s phthalimide synthesis:
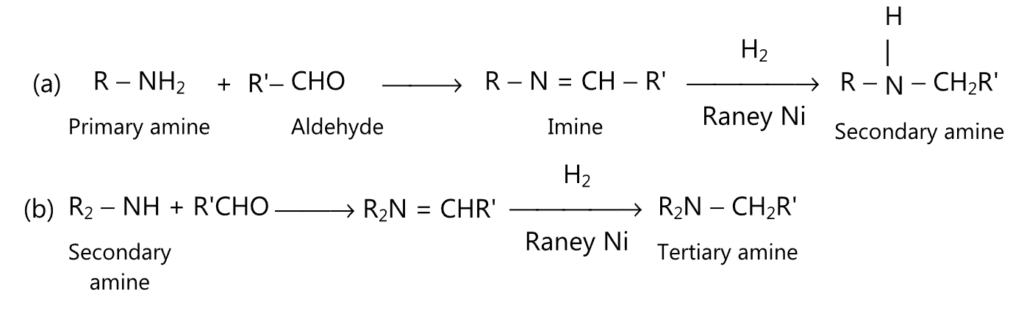
(viii) Henze reaction (for secondary and tertiary amine):
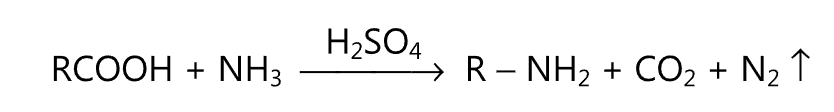
(ix) Decarboxylation of amino acids:
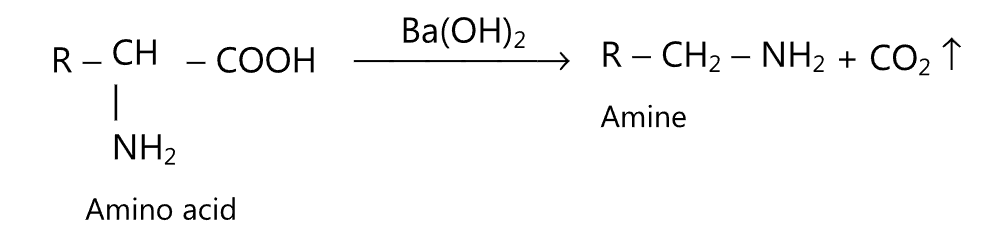
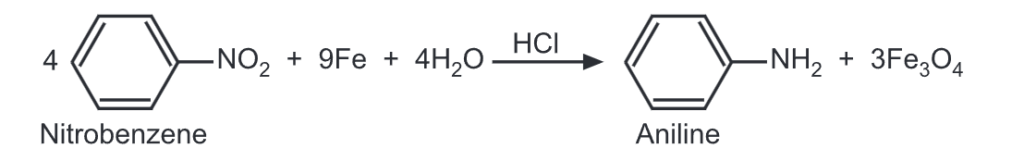
The preparation of aromatic amines does not differ principally from the methods used for aliphatic amines. For example, the reduction of a nitro compound may be carried out either by catalytic hydrogenation or by the reagents like iron and a dilute HCI.
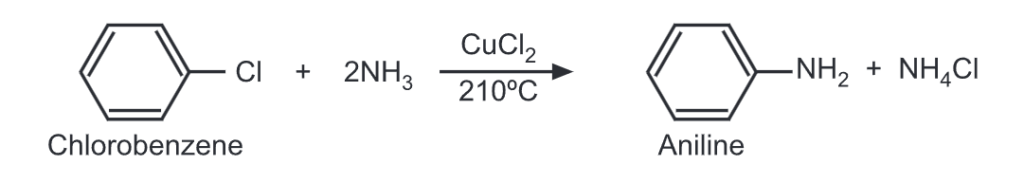
Similarly, a chloro compound or phenol may be treated with ammonia (i.e. ammonolysis) at high temperature and high pressure in the presence of a catalyst to get aromatic amine.
Hofmann rearrangement and many other rearrangement reactions specified in the preparation of aliphatic amines are equally applicable in the preparation of aromatic amines.
Make sure you also check our other amazing Article on : Structure and Uses of Phenol