Aim: To prepare 100 ml of a pharmaceutical buffer solution of pH 5.0 (acetate buffer) and verify the same by measuring pH using a pH meter.
Principle of Buffer Solution
Buffers are compounds or mixtures of compounds; when present in solution, resist change of pH upon the addition of a small quantity of acid or alkali. Such solutions are called buffer solutions and are usually solutions of weak acids (or bases) and their corresponding salts (acetic acid and sodium acetate; ammonia and ammonium chloride).
The buffer equation is utilized to find out the quantity of weak acid and salt; or weak base and salt; required to get a buffer solution of desired pH. The buffer equation (also known as Henderson – Hasselbalch equation):
For weak acid and its salt:
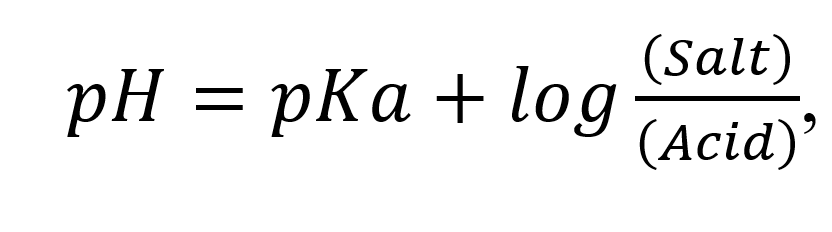
, and for acetate buffer of pH 5.0, the equation is:
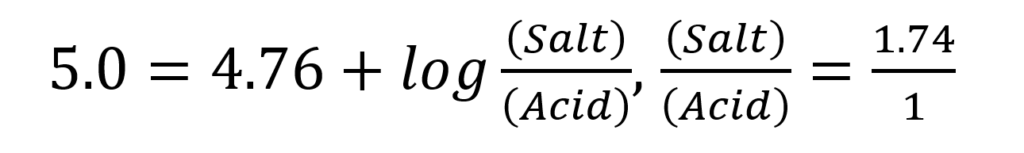
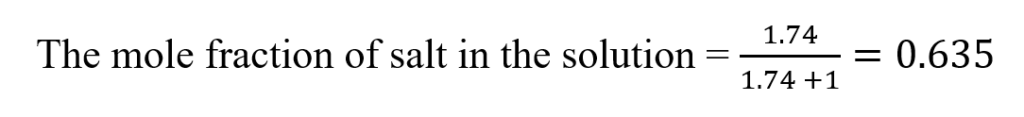
The mole fraction of salt in the solution =
The concentration of salt in the buffer solution (mole fraction) = 63.5%
The concentration of acid in the buffer solution (mole fraction) = 36.5%
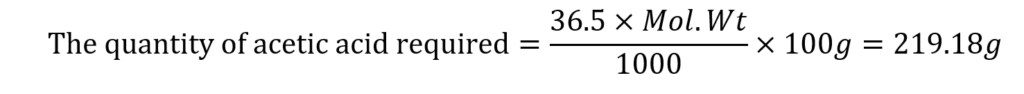
(the molecular weight of acetic acid is 60.05)
Then the weight of acetic acid is converted to volume using weight per ml data.
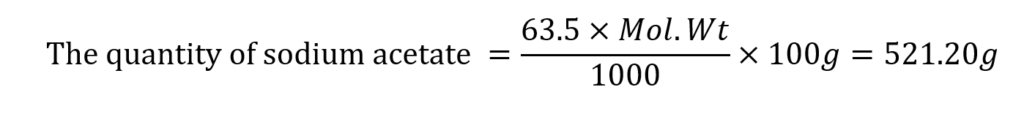
(The molecular weight of sodium acetate is 82.08)
Apparatus and materials required: Standard buffers, Acetic acid, Sodium acetate, and pH meter.
Preparation of Buffer Solution
Process:
- The quantity of acetic acid and sodium acetate are dissolved in around 90 ml of distilled water and the volume is adjusted to 100 ml.
- The pH meter is calibrated using a standard buffer.
- The pH of the buffer solution is recorded.
Observation and Calculation:
Room temperature: ________0C
The pH of the prepared buffer solution is _______
Report: The prepared buffer solution has pH ____________
Make sure you also check our other amazing Article on: Determination of Critical Micelle Concentration
