Corrosion is a natural process, it can be controlled by using the prevention of corrosion methods.
Table of Contents
Selection of Proper Material
Corrosion should not be permitted in fine mesh wire screens, orifices, and other items in which dimensions are critical and changes are not permitted. In many cases, non-metallic materials will be useful and attractive from the point of view of economics and performance. These should be considered if their strength, temperature, and design specifications are satisfactory.
The corrosion characteristics of chemicals and limitations of construction materials must be considered in the literature before selecting equipment. In addition, processing conditions to which the material is exposed should also be considered. For this purpose, relevant literature should be consulted. Permissible corrosion rates are important factors and differ with equipment. Appreciable corrosion can be permitted for tanks and lines if anticipated and allowed for thickness in its design.
Proper Design of Equipment
In the design of equipment, a number of fittings such as baffles, stiffeners, drain nozzles, location of valves and pumps should be considered. Corrosion can be minimized if the equipment design facilitates it.
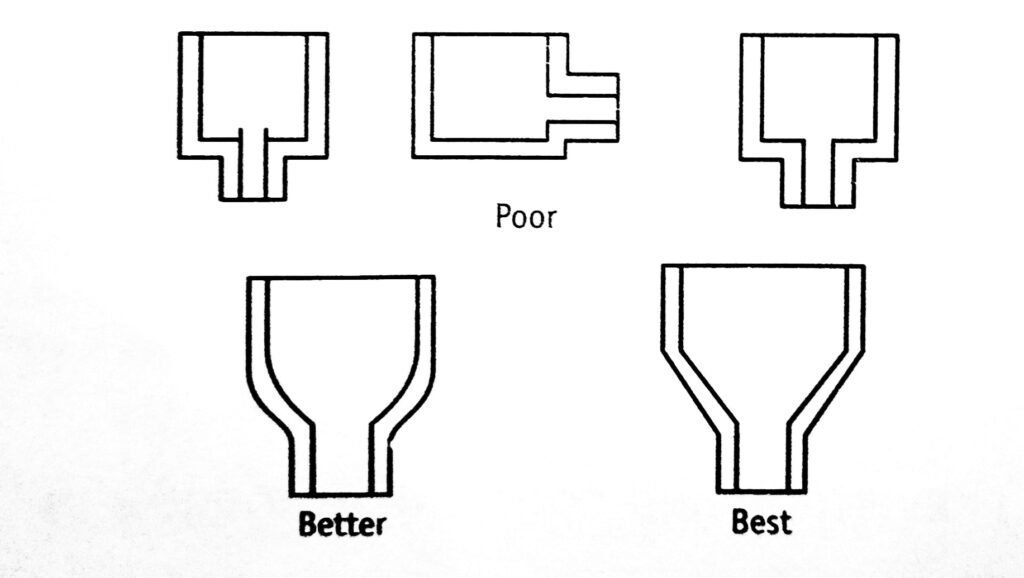
- Elimination of crevices.
- Complete drainage of liquids.
- Ease of cleaning.
- Ease of inspection and maintenance.
On the basis of these general principles, some poor and good engineering designs are given in Figure 1.1. Direct contact between two metals should be avoided if they are separated widely in the electrochemical series. Otherwise, they should be insulated. Equipment should be supported in such a way that it will not rest in pools of liquid or on the damp insulating material.
Coatings and Linings
Nonmetallic coatings and linings can be applied to steel and other materials of construction in order to combat corrosion. Appropriate methods such as electroplating, cladding, and organic coatings should be considered. The thickness of the linings is important (100 mils). Effective linings can be obtained by bonding directly to substrate metal or building multiple layers or lamination.
Organic coatings are used as linings in equipment such as tanks, piping, pumping lines, and shipping containers. Some examples of the linings are:
- Ceramic
- Carbon brick
- Plastic
- Elastic
- Glass-coated
- Organic
A thin non-reinforced paint-like coating of less than 0.75 mm thickness should not be used in services for which full protection is required from corrosion. Some examples of linings and uses are:
| Linings | Uses |
| Tin coated steel (tin plate) | Food containers |
| Lead (Pd) coating (terne plate) | roofings |
| Aluminum (Al) coated steel | High temp conditions |
The cladding (i.e., mechanical bonding) of steel with an alloy is another approach to this problem. For instance, special glasses can be bonded to steel so that the liner is 1.5 mm thick which is impervious. Equipment and piping are lined in this manner and routinely used in severely corrosive acid services. The only limitation of this method is the service behavior of the protective coatings.
Altering Environment
Corrosion can be combated or reduced by employing the following environmental conditions.
- Removing air from boiler feed water prevents the influence of water on steel.
- Reducing aeration prevents the formation of the passive oxide film in stainless steel alloys by acidic media.
- Pumping of inert gas into solutions prevents the contact of air or oxygen as in the case of nickel-based alloys.
- Reducing the temperature.
- Eliminating the moisture.
- Reducing the velocity or turbulence.
- Shortening the time of exposure.
The addition of acid media should be done as a last step, so that maximum dilution can be obtained.
Inhibitors
The corrosion inhibitors are added to the environment to effectively decrease the corrosion of metals. These form protective films.
Adsorption type, for example, adsorbed on the metal. Scavenger types, for example, remove corrosion agents. Vapour phase-type, for example, sublime and condense on the metal surface.
Examples of inhibitors are given below.
Inhibitors also interfere with the process of corrosion, namely scavenging oxygen, and hydrogen, and reducing the diffusion of the constituents.
- The diffusion of H+ ions is considerably decreased by organic inhibitors. Examples are amines, mercaptans, substituted ureas, and thioureas. These are cathodic inhibitors.
- Reducing agents eliminate oxygen from the corroding medium. An example is sodium sulfite.
- Inhibitors retard the diffusion to the cathodic areas. Examples are magnesium, zinc, and nickel salts.
Inhibitors are generally used in quantities less than 0.1% by weight. In some cases, the amount of inhibitor used is critical.
Cathodic Protection
The cathodic protection is based on the galvanic action between the metal(s) of the plant (cathode) and the anode suspended in the solution. The metal to be protected is made a cathode, i.e., electrons are supplied, thereby dissolution of metal is suppressed. This can be achieved by two methods.
- Sacrificial anode method
- Impressed emf method
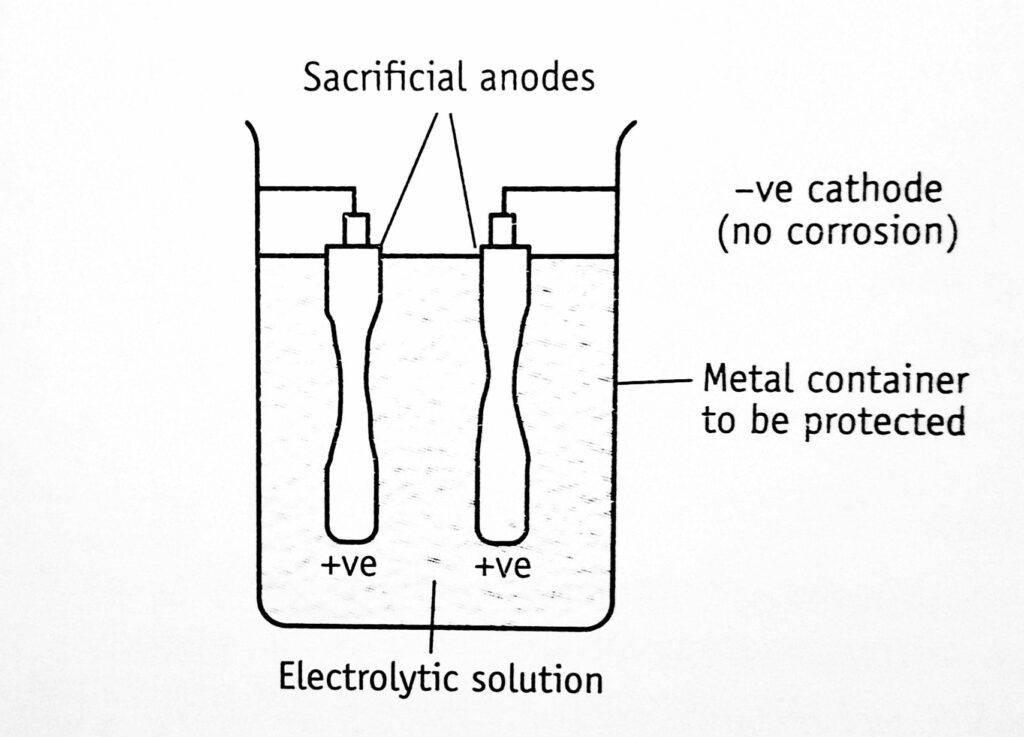
Sacrificial anode method: In this method, anodes are kept in electrical contact with the metal to be protected (cathode). The anodes are sacrificed since it goes into the solution (Figure 1.2). For example, for the protection of iron and steel tanks, the metals such as zinc, aluminum, and magnesium and their alloys are used as sacrificial anodes. These are used in a limited pH range when a high solution rate is acceptable since they are amphoteric.
Anode metal is selected from the electrochemical series amongst the metals present above the tank metal. The anode should not be poisonous and not detrimental to the product.
Impressed emf method: This is also known as the applied current system, i.e., the external voltage is impressed between tank and electrodes. The negative terminal of the power supply is connected to the material to be protected. Therefore, the natural galvanic effect is avoided and the anode is maintained positive (Figure 1.3)
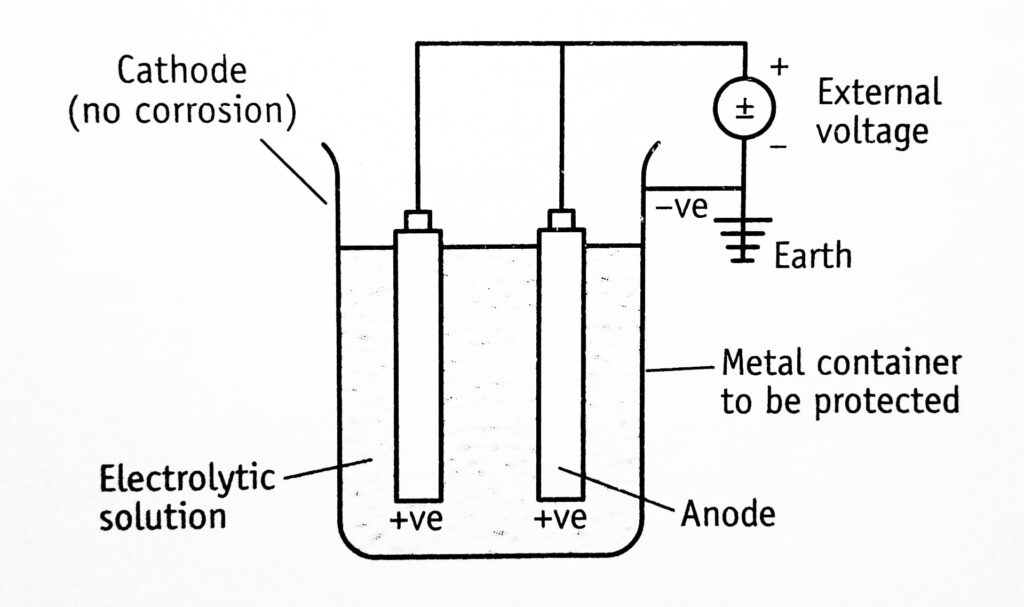
The term impressed emf (or current) means the current which is greater than the corrosion current. When such an impressed current is applied in opposite direction, the corrosion effect is nullified. Under such conditions, the anode is converted to the cathode in the corroding metal. Since anode is not consumed, any conducting material, metal, or non-corrodable alloys can be used. For example, in the case of sulphuric acid and deionized water, graphite and high silicon steel are impressed. The anodes can be buried in the ground or suspended in the aqueous solution of electrolyte.
Advantages:
- This method is used for large tanks to store mild corrosive liquors. In these cases, mild steel is used with negligible corrosion.
- The cathodic protection method is simple and the most effective.
- It is inexpensive. It enables the use of cheaper materials for plant construction.
Disadvantage: Corrosion cannot be reduced to zero.
Anodic Protection
In the method, a predetermined potential is applied to the metal specimen and the corresponding current changes are observed. During the initial stage, the current increases indicating the dissolution (corrosion) of the metal. When the current reaches a critical point, passivization occurs, i.e… the oxide layer sets in a suitable oxidizing environment. The potential at the critical point is called passivating potential. Above this passivating potential, the current flow decreases to a very small value called passivating current. The passivating current is defined as the minimum protective current density required to maintain passivization.
Passivity is a phenomenon in which a metal or alloy exhibits much higher corrosion resistance than expected from its position in the galvanic cell. At this stage, an increase in potential will not corrode the metal since the latter is in a highly passive state. For example, in the case of stainless steel, titanium becomes easily passive and cannot offer cathodic protection. In such cases, the corrosion rate may be slowed down by the use of an anodic current.
Advantage: Anodic protection requires a small current. The anodic protection method is utilized in the transportation of concentrated sulphuric acid.
Disadvantages:
- Corrosion cannot be reduced to zero.
- This method cannot be applied to metals, which do not passivate. A proper material should be selected for a specific process based on the literature and personal experience. The factors influencing corrosion will not only help in selecting the right kind of material but also suggest the processing conditions. It is equally essential to identify the type of corrosion if it occurs. Since the theories of corrosion are known, it is possible to adopt appropriate preventive measures.
Make sure you also check our other amazing Article on : Theories of Corrosion
