The metal surface undergoes an electrochemical reaction with the moisture and oxygen in the atmosphere. These Theories are known as the electrochemical theory of Corrosion. The mechanism involves the formation of a galvanic cell (anodic and cathodic areas), by different metals (for example, Fe and Cu) or in different areas on the same piece of metal (for example, iron).
When galvanic cells are formed on different metals, the corrosion is known as galvanic corrosion. These reactions are illustrated using metals in the presence of electrolyte solutions such as hydrochloric acid.
Corrosion Reactions on Single Metal
Electrochemical reactions are illustrated by considering the corrosion of a piece of iron in hydrochloric acid. Anodic and cathodic areas are formed on the surface of iron, owing to surface imperfections (localized stresses, grain orientation, inclusions in the metals) or due to variations in the environment. Numerous tiny reactions may occur (Figure 1.1).
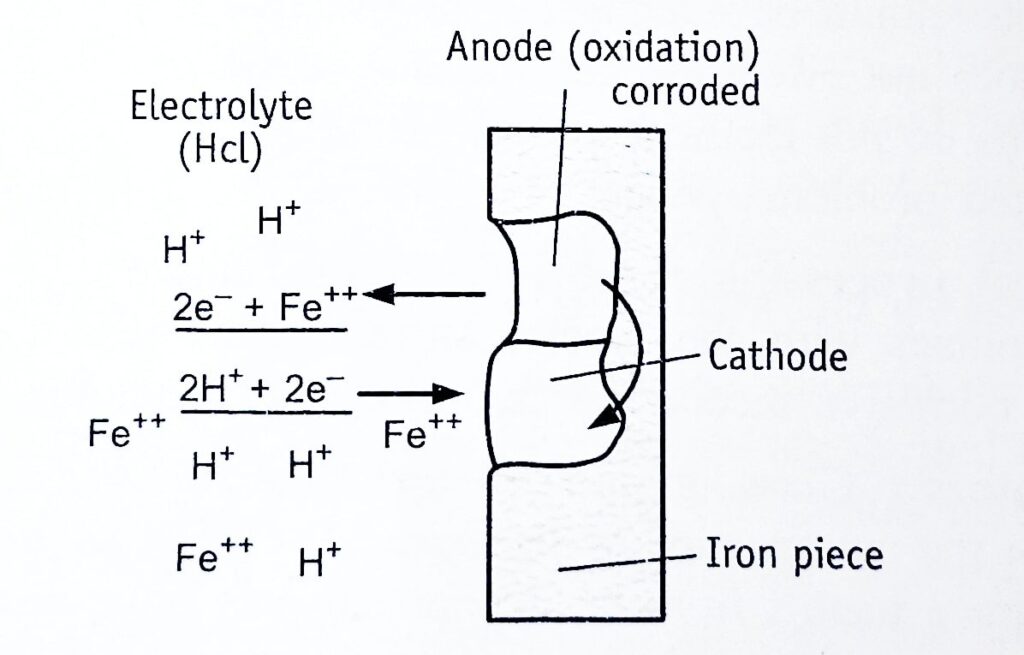
Reaction at the anode: Oxidation takes place with the release of electrons. Positively charged iron atoms get detached from the solid surface and enter into the solution (electrolyte) as positive ions.
At anode (oxidation): Fe——> Fe++ 2e– (indicated by rough surface)
The released free electrons (negative charge) pass round the external circuit.
Reaction at the cathode: Reduction of constituents occurs with the taking up of electrons. The free electrons reach the cathode and react with some positively charged species such as hydrogen ions in the electrolyte solution. In the absence of acid, water itself dissociates to generate H+ ions.
At cathode (reduction): 2H+ + 2e– ——> H2↑ (indicated by formation of bubbles at the surface)
The amount of metal (iron) that is dissolved in the electrolyte is proportional to the number of electrons flowing, which in turn is dependent upon the potential and resistance of the metal.
The overall reaction:
Fe + 2H2O——–> Fe(OH)2 + H₂ (increases) + H₂ (increases) Red brown rust
High evolution of H2 accompanies rapid corrosion such as hydrogen embrittlement. In the electrochemical series, all metals above hydrogen have a tendency to get dissolved in an acidic solution with simultaneous evolution of oxygen. Depletion of hydrogen also enhances corrosion. In moderate concentrations of H2, corrosion slows down.
Corrosion Reactions Between Metals
Galvanic corrosion results from the flow of current from a more active metal (anode) to a less active metal (cathode). A few examples are steel pipe connected to copper plumbing and lead-antimony solder around the copper wire.
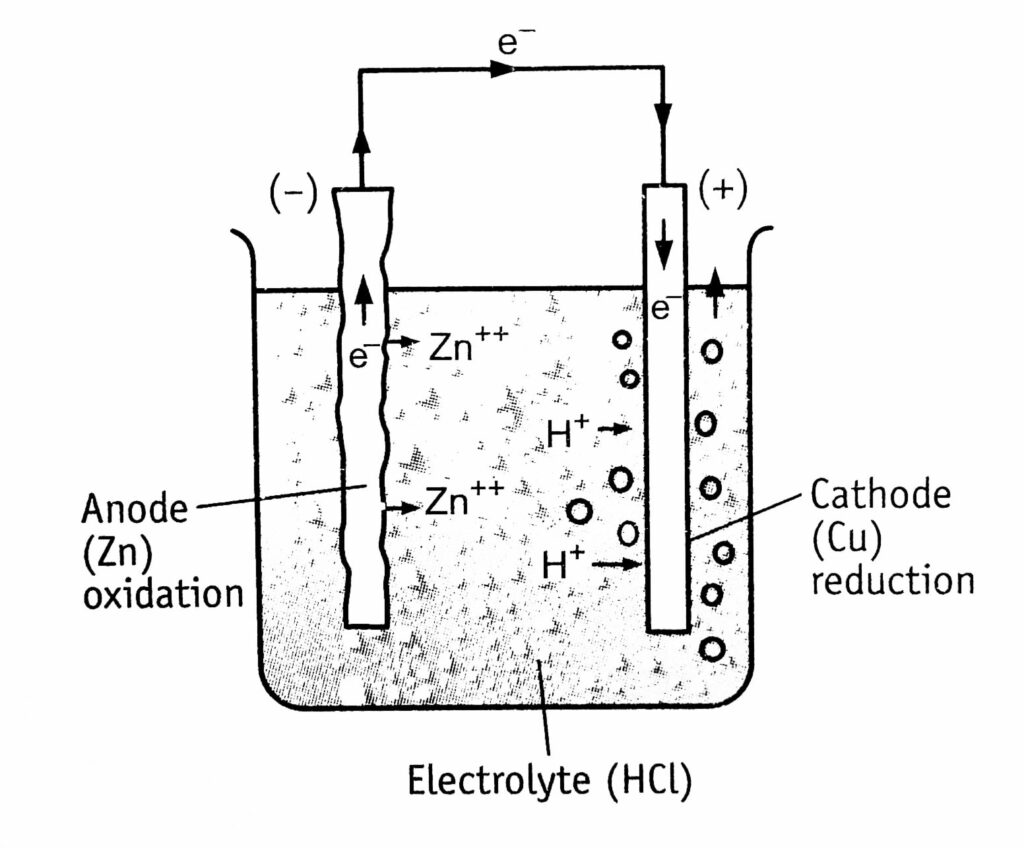
For example, zinc dissolves and forms an anode, while copper (Cu) forms the cathode. These two metals form two electrodes and their presence in an electrolytic solution forms a galvanic cell (Figure 1.2).
The spontaneous reaction can occur when two electrodes are connected through an external wire. Reactions at anode and cathode are:
At anode (oxidation): Zn——–> Zn+++ 2 e– (indicated by rough surface)
At cathode (reduction): 2H+ + 2e–———> H₂ (increases) (indicated by formation of bubbles at the surface)
The corrosion current flows at the expense of the anode metal, which gets corroded continuously, whereas the cathode metal is protected.
In some cases, the evolution of hydrogen gas is slow. The accumulation of a layer of hydrogen on the cathode surface slows down the corrosion. This is called cathodic polarisation. It forms an insulating layer that slows down or stops the electrochemical reaction.
Corrosion Involving Oxygen
The oxygen dissolved in an electrolyte can react with accumulated hydrogen to form water. Depletion of the hydrogen layer allows corrosion to proceed.
At cathode: O2 + 2H2———> 2H2O (indicated by the formation of bubbles at the surface)
The above reaction takes place in acid media. An example is rusting of iron. The surface of iron is usually coated with a thin layer of iron oxide. However, if this iron oxide film develops a crack, anodic and cathodic areas are created. When the corrosion media is alkaline or neutral, oxygen is absorbed. The presence of moisture (or water) promotes corrosion. The effective concentration of oxygen in water adjacent to the cathode depends upon the degree of aeration, temperature, and presence of dissolved salts.
Make sure you also check our other amazing Article on : Simple Distillation
