By heating the hydrazone of a carbonyl compound in a sealed tube with sodium ethoxide or sodium hydroxide as a catalyst, nitrogen is evolved and the corresponding methylene compound is formed. This method of reduction is called Wolff-Kishner reduction.
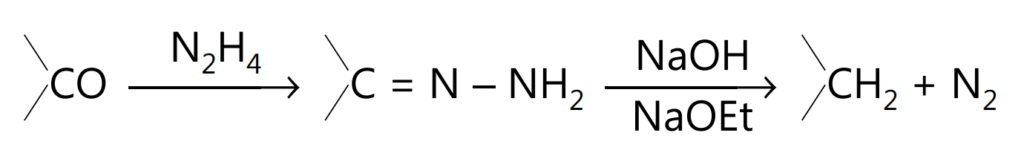
The semicarbazone and ozine derivatives have also been employed. In both cases, conversion to hydrazone is essential before reduction occurs.
This method is suitable for the reduction of high molecular weight carbonyl compounds as well as those sensitive to acid. The Clemmenson reduction which is more commonly employed for the reduction of aldehydes and ketones is not satisfactory for these types of compounds. Furthermore, it has been shown with some α amino ketones, the expected product is obtained by Wolff-Kishner reduction whereas anomalous products are formed via Clemmenson’s method.
The reaction as originally carried out by Wolff consists of heating the hydrazone with sodium ethoxide in a sealed tube at about 180oC for several hours.
Mechanism
The classical Wolff-Kishner decomposition of the hydrazones of aldehydes and ketones in an alkaline medium at high temperatures to give hydrocarbons involves anion formation followed by isomerization to an azo compound and loss of nitrogen as shown below.

The severity of the reaction condition can be brought down to room temperature if a strong base is used in a highly polar medium.
Modifications of Wolff-Kishner Reduction:
Huang Minlon Procedure: The ketone to be reduced (1 mole) and 3 moles of hydrazine hydrate are added to a solution of KOH, 3 moles in diethylene glycol, which has been cooled below 100oC. This mixture is heated cautiously until the initial exothermic reaction is complete and is then gently refluxed for 1 hr. At this stage, water and hydrazine are distilled from the reaction mixture until the temperature of the liquid reaches 200 − 210oC. Refluxing for 3 − 5 hrs. is continued at 200oC. This appears to be the most satisfactory modification of Wolff-Kishner reduction. By this procedure, phenoxy benzoyl propionic acid was reduced by 95% yield to phenoxy phenyl butyric acid. This reduction by Clemmenson gave a 54% yield.

The Huang Minlon modification is applicable to large as well as small-scale reductions. With aryl methyl ethers, demethylation usually occurs with Huang Minlon modification. Oxidation of resulting phenol is avoided by using an atmosphere of nitrogen and a shortened reaction period. The reducing power of the reaction is increased considerably by using strictly anhydrous conditions. Sterically hindered carbonyl compounds may be reduced in this manner.
Lower Temperature Modification: Gates and Tschudi have pointed out that reduction will often proceed well at temperatures lower than those employed in Huang Minlon. Veratraldehyde has been reduced to 4-methyl veratrole in excellent yield by heating a DEG solution of the reactants for 3 hours at 135oC.
Two-Step (Lock modification): The Huang Minlon is often improved (particularly if base-sensitive carbonyl compounds are to be reduced) by reacting the substrate with hydrazine hydrate for a short period before the base is added.
Barton modification: Sterically hindered ketones are resistant to Huang Minlon reduction but will often respond to vigorous treatment with anhydrous hydrazine.
Nagata Itazaki: A reduction procedure that appears to be more vigorous than Barton and which avoids the use of anhydrous hydrazine.
Two variations are:
- A mixture of 0.01 moles of the substrate, 0.66 moles of hydrazine hydrate, 0.08 moles of hydrazine dihydrochloride, and 1.5 moles of triethylene glycol are heated at 130oC for 2.5 hours. After adding 0.2 moles of KOH pellets, the temperature was raised to 210oC. While the low boiling materials are distilled, heating was continued at 210oC for 3 − 4 hours.
- A mixture of 0.01 moles of the substrate, 4.0 moles of hydrazine, 0.3 moles of hydrazine dihydrochloride, and 1.5 moles of triethylene glycol is heated at 130oC for 7 hours. After adding 0.7 moles of KOH, the temperature was raised to 220oC as before.
Gram modification: Studies of anion reactions in Dimethyl sulfoxide solution have shown a remarkable rate enhancement when the Wolff-Kishner reduction is carried out in that solvent.
Applications of Wolff-Kishner Reduction
(1) Reduction of camphor to camphane:

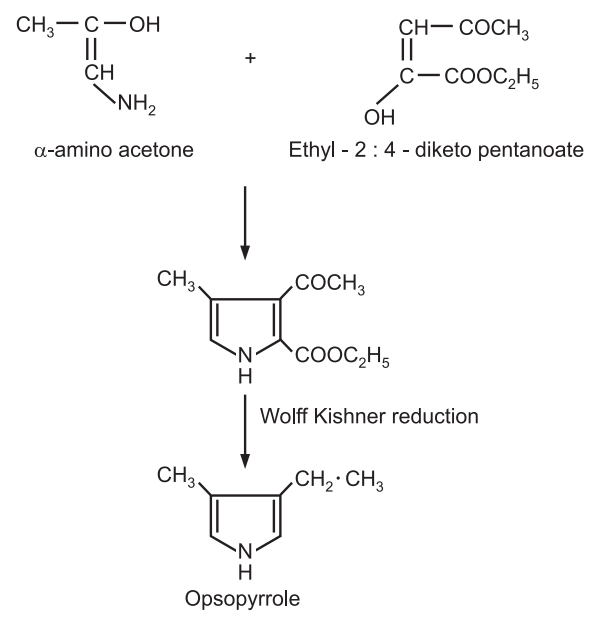
(2) Synthesis of pyrroles: Structures of pyrroles (degraded products of haemin and chlorophyll) can be proved by their synthesis which involves Wolff-Kishner reduction. e.g. synthesis of opsopyrrole.
(3) Elucidating the structure of estrone: Estrone methyl ether on Wolff-Kishner reduction followed by selenium dehydrogenation gives a compound 7 methoxy – 1, 2-cyclopentenophenanthrene of known structure.
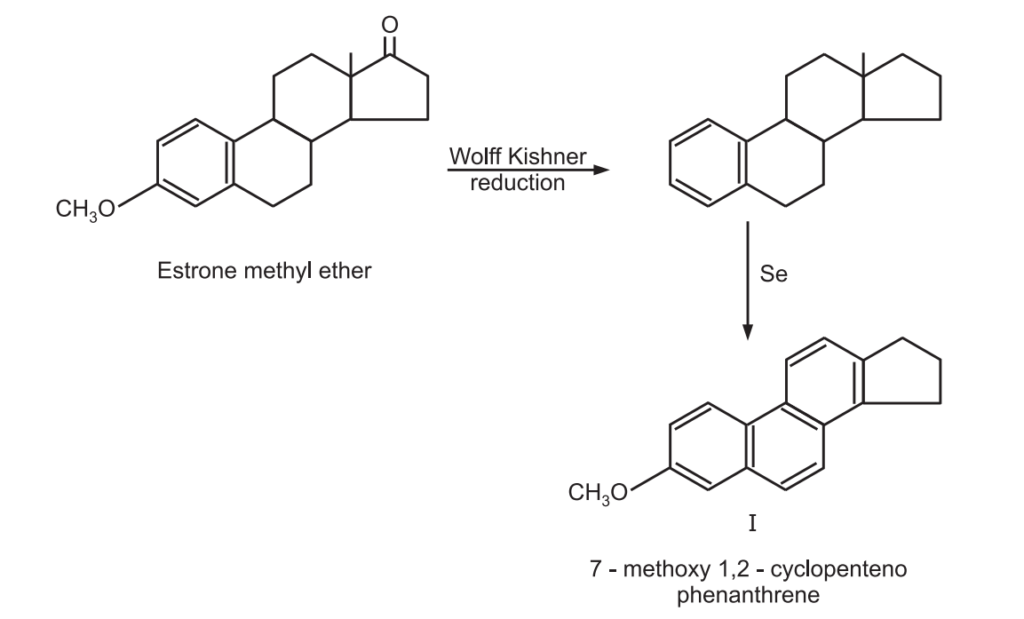
The structure of the degraded product (I) clearly indicates the carbon skeleton of estrone. Moreover, it also establishes the position of the phenolic group in estrone.
Reduction:
Reduction involves:
- the removal of oxygen,
- the addition of hydrogen, and
- the gain of electrons.
The addition of hydrogen may be subdivided into
(1) Hydrogenation i.e. the addition of hydrogen to an unsaturated system

(2) Hydrogenolysis is the addition of hydrogen with concomitant bond repture

Make sure you also check our other amazing Article on : Thiophene Reactions