The reaction of Phenols: Phenol undergoes electrophilic aromatic substitution reactions. Due to the electron-donating nature of the OH group, the next substitution takes place preferably at ortho and/or para positions to the -OH group.
The reaction of Phenols:
(a) Bromination: An aqueous bromine solution brominates all ortho and para positions on the ring to give a white precipitate of 2, 4, 6-tribromophenol.
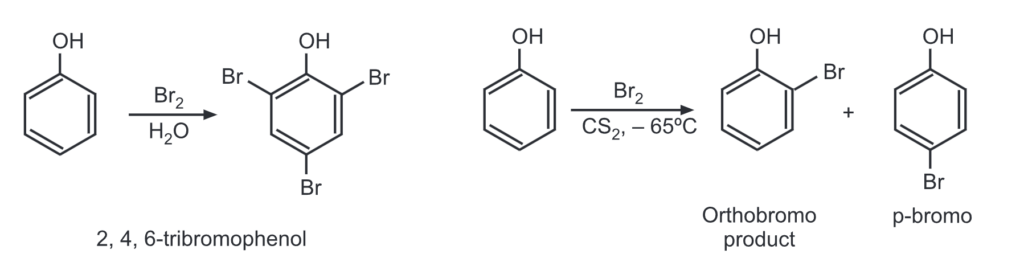
Monobromination is done by running the reaction at an extremely low temperature in carbon solvent.
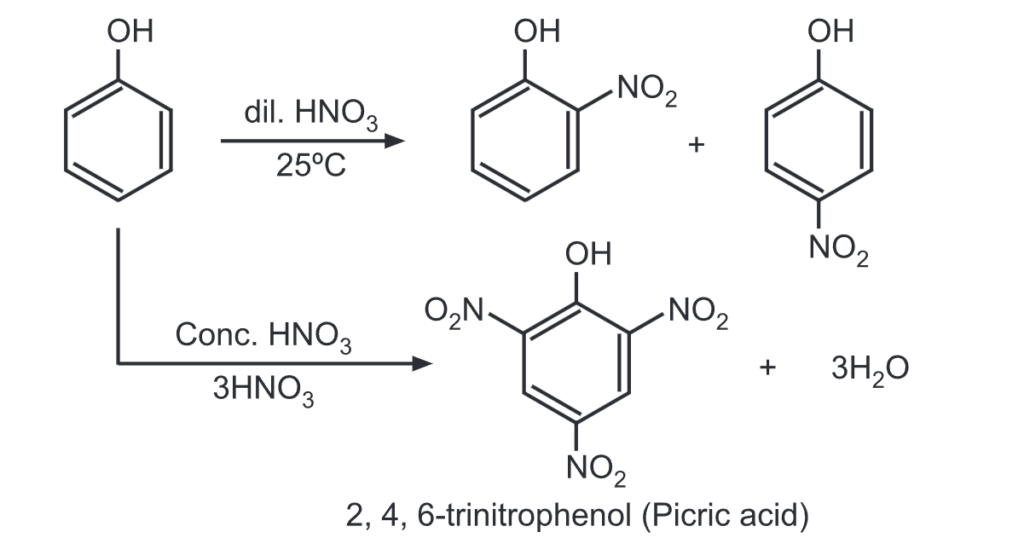
(b) Nitration: Phenol, when treated with dil. HNO3 at room temperature gives mono-nitrated derivatives. While 2, 4, and 6-trinitrophenol is obtained when phenol is treated with conc. HNO3.
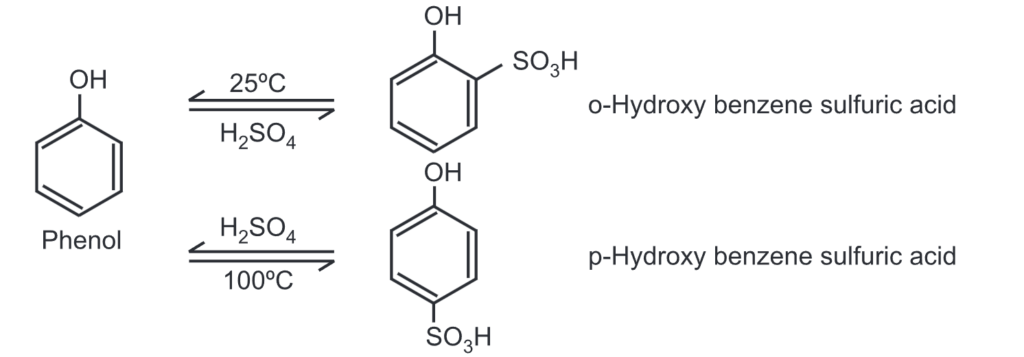
(c) Sulfonation: Phenol reacts with conc. H2SO4 At 25°C, the ortho product predominates while at 100°C, the para product is the major product.
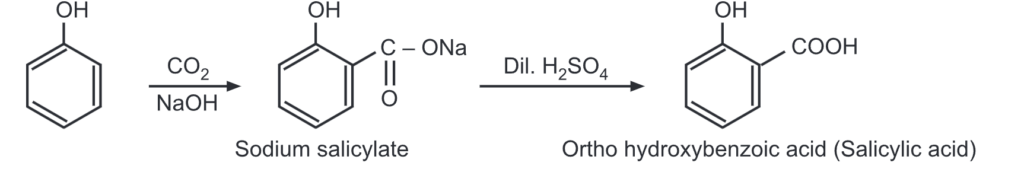
(d) Kolbe’s reaction: Phenol reacts with a weak electrophile i.e., CO2 in basic conditions to give o-hydroxybenzoic acid.
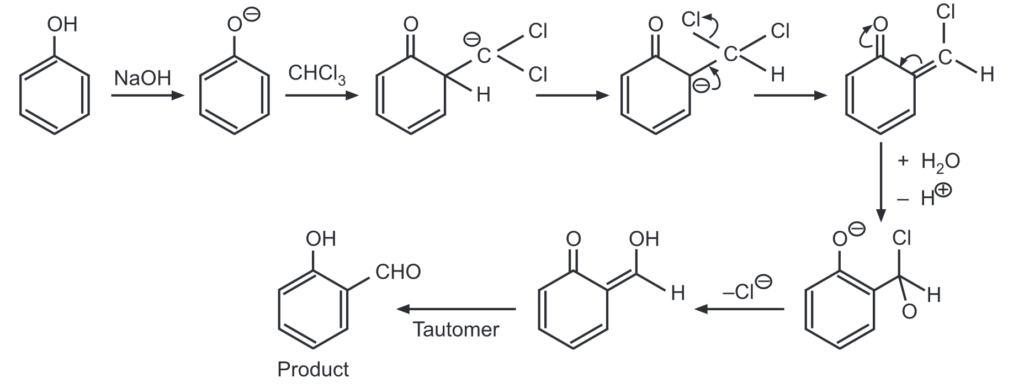
(e) Reimer-Tiemann reaction: Phenol reacts with chloroform in the presence of sodium hydroxide to give ortho hydroxy benzaldehyde.
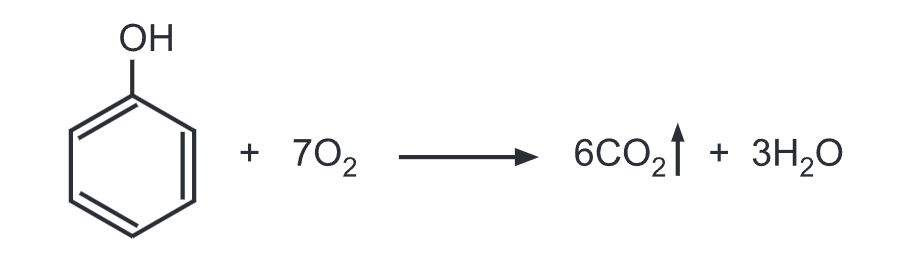
(f) Combustion of phenol: Phenol burns in presence of oxygen to give carbon dioxide (CO2) and water. Phenol tends to burn in air with an extremely smoky flame due to the high proportion of carbon in phenol.
(g) Reduction of phenol: Phenol is reduced to benzene when it is distilled with zinc dust or when phenol vapor is passed over granules of zinc at 400°C.

(h) Phenol reacts with diazomethane in the presence of boron trifluoride (BF3) to give anisole.
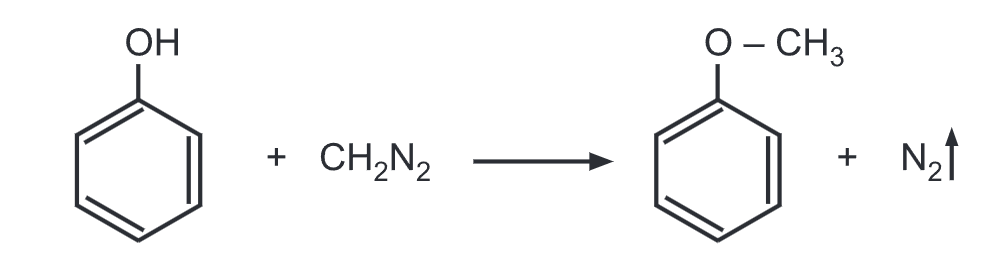
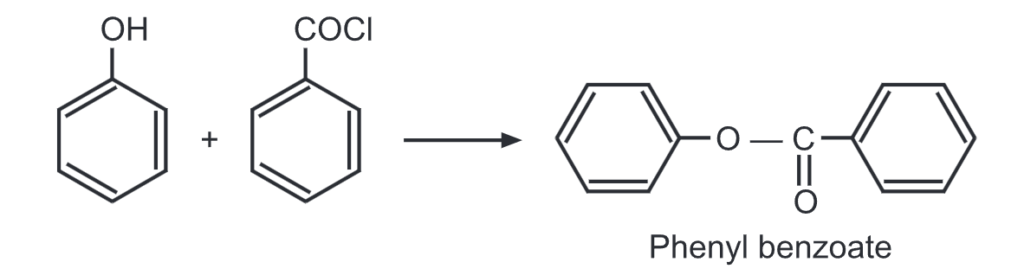
(i) Esterification of phenol: Unlike alcohols, phenol reacts with a carboxylic acid at a very slow rate. Hence, highly reactive forms of carboxylic acids like acid chlorides/acid anhydrides are used instead of carboxylic acids. For example,
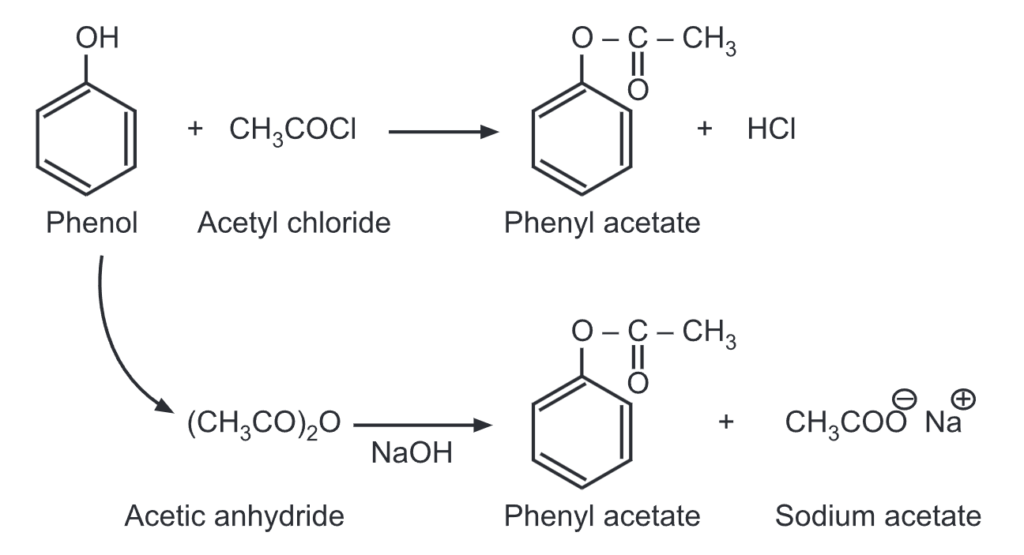
When a mixture of phenol and benzoyl chloride is shaken in the presence of dil. NaOH solution, phenyl benzoate is formed. This is an example of the Schotten-Baumann reaction.
Make sure you also check our other amazing Article on : Methods of Preparation of Phenols
