Reactions Of Carboxylic Acid: The reactivity of both, aliphatic and aromatic carboxylic acids is due to the presence of a carboxyl (-COOH) group. The carboxyl group contains both, carbonyl (-C=O) and alcoholic (-OH) functions. The reactions due to the carbonyl group lead to the formation of acid derivatives while reactions involving the remainder of the molecules result in the substituted acids.
General Reactions of the Carboxylic Acid
(a) Neutralisation: Because of the acidic properties, carboxylic acids form salts upon neutralization by alkalies.
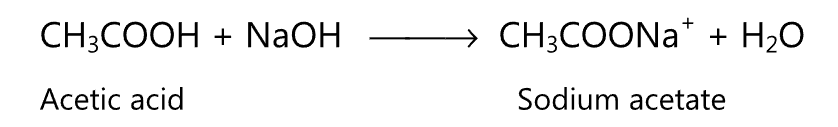
(b) Reactions due to the carbonyl (C=O) group: Upon catalytic hydrogenation or with lithium aluminum hydride, carboxylic acids may be converted to corresponding alcohols by the replacement of carbonyl oxygen with hydrogen atoms.

Catalytic hydrogenation at high temperatures and under high pressure may lead to the formation of alkane from a carboxylic acid.
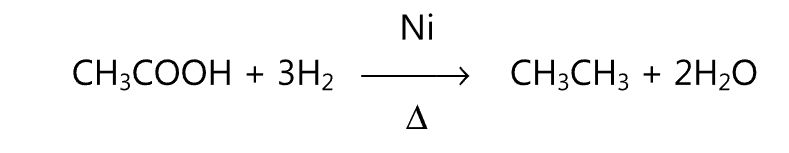
Similarly, the alkane is obtained when the anhydrous sodium salt of a carboxylic acid is heated with soda lime.
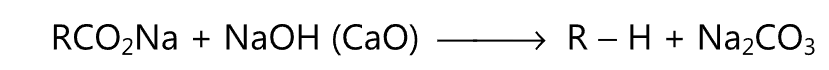
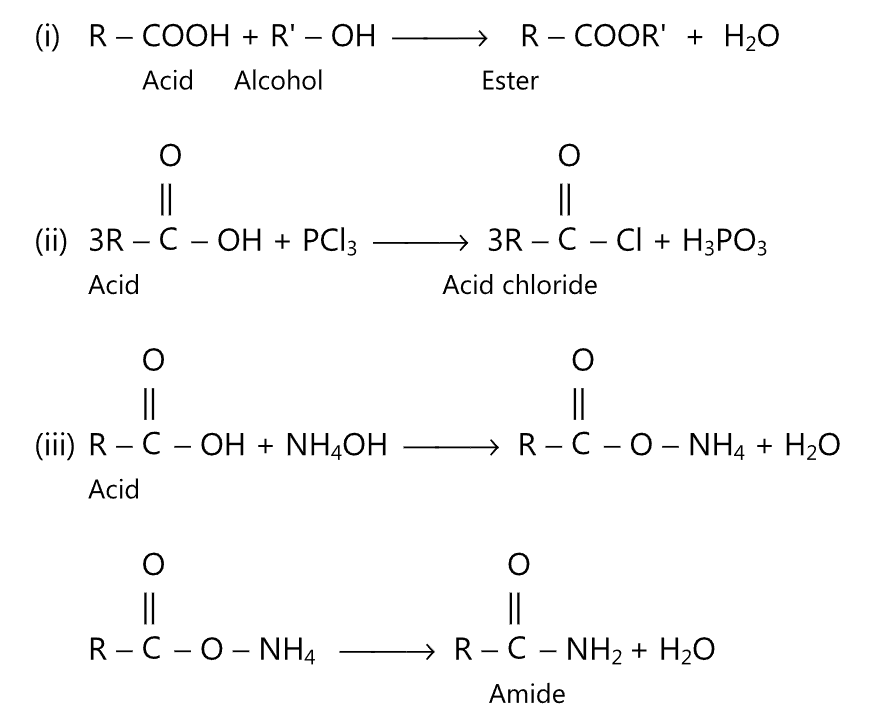
(c) Reactions due to the -OH group: The hydroxyl group of the carboxyl moiety may be replaced by alkoxy, halogen, or amine group to give ester, acid chloride, and amide respectively.
For example,
(d) Miscellaneous reactions:
(i) With the exception of formic acid, carboxylic acids are extremely resistant to oxidation. However, upon prolonged heating with an oxidizing agent, carboxylic acid produces carbon dioxide and water as the end products.
(ii) α-Halogen acids are obtained when carboxylic acid is heated with chlorine or bromine in the presence of a small amount of phosphorus.
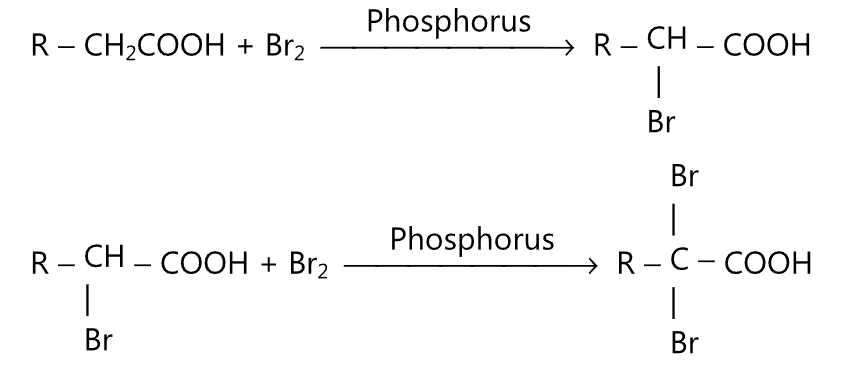
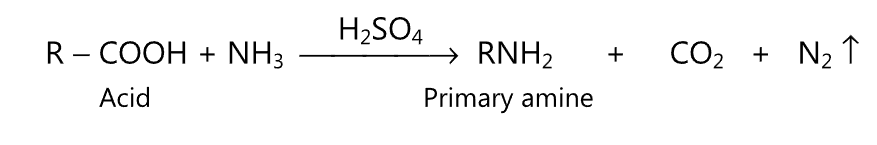
(iii) In the presence of sulphuric acid, the carboxylic acid is converted to a primary amine.
Make sure you also check our other amazing Article on : Synthetic Uses of Aryl Diazonium Salts
