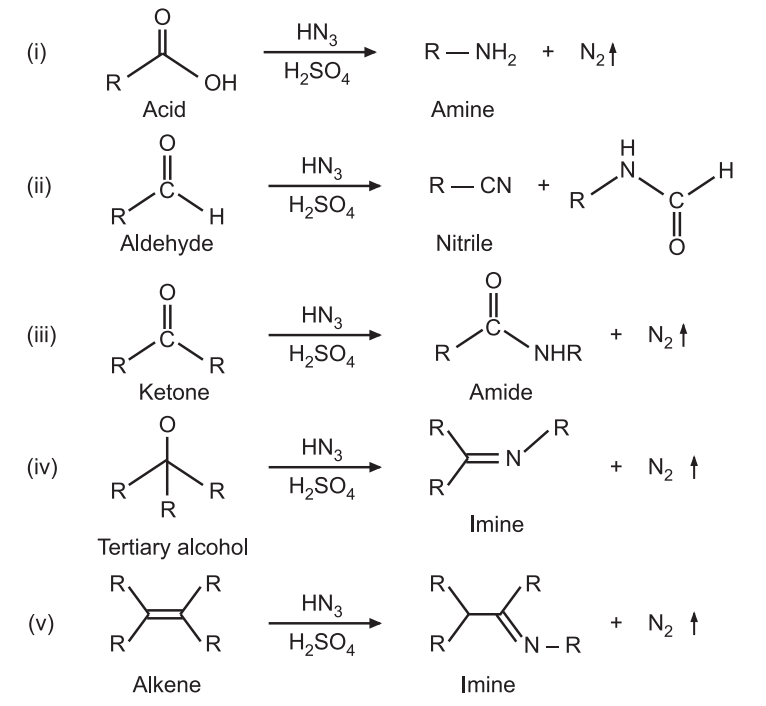
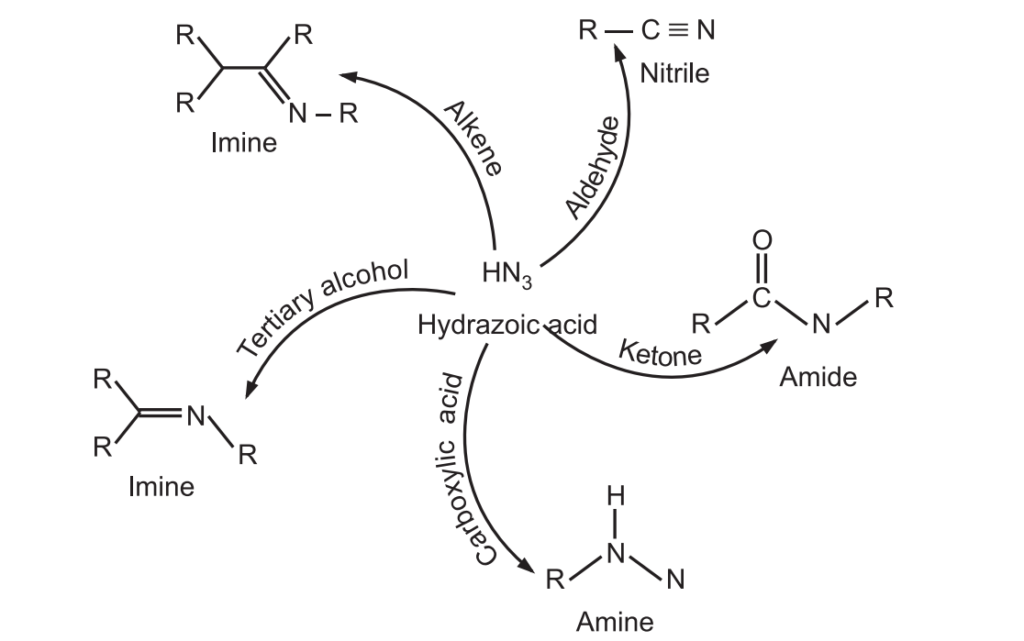
Schmidt Rearrangement reaction was discovered by Karl Friedrich Schmidt in 1924. Azides (RN3) are nucleophilic at their terminal nitrogen atoms and may add to suitably activated electrophiles, such as carbonyl compounds, tertiary alcohols, or alkenes to give amines, nitriles, amides, or imines. It is an acid-catalyzed reaction. For example:
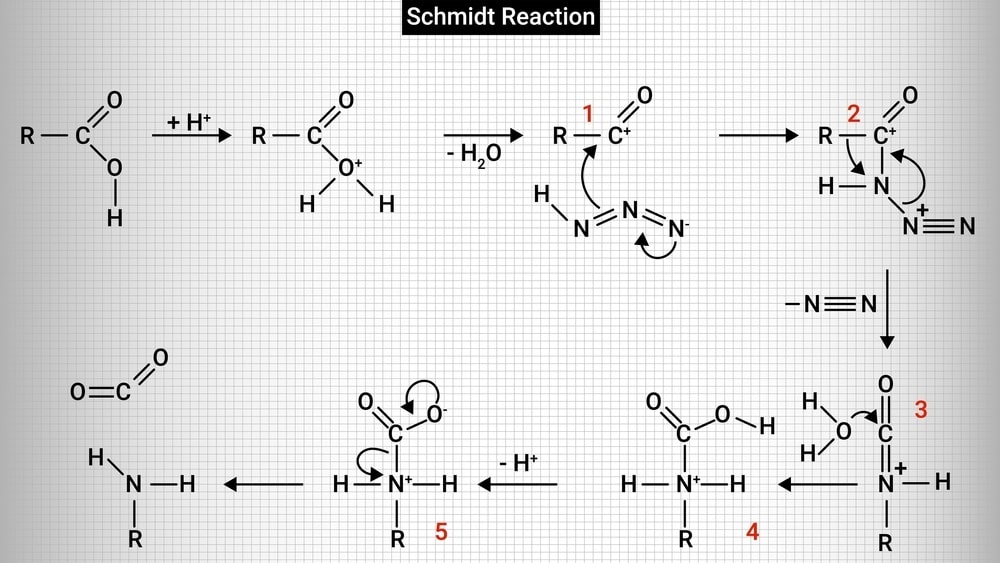
Reaction mechanism:
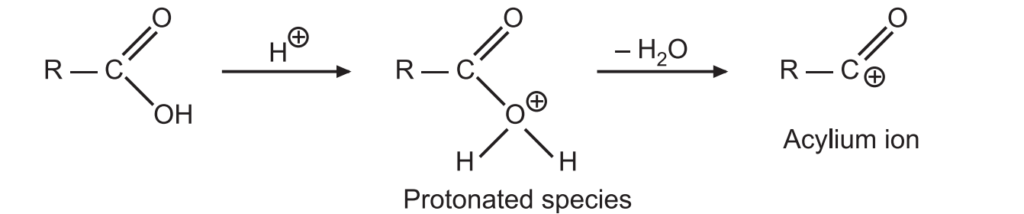
1. Initially, a protonation reaction takes place and a water molecule is lost forming an acylium ion.
2. The acylium ion then reacts with hydrazoic acid to form protonated azido ketone. The alkyl group shifts from carbonyl carbon to the nitrogen atom by rearrangement reaction.
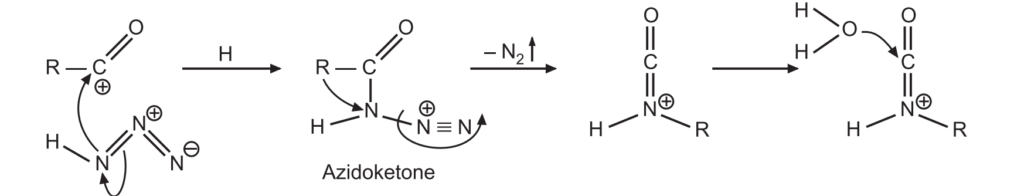
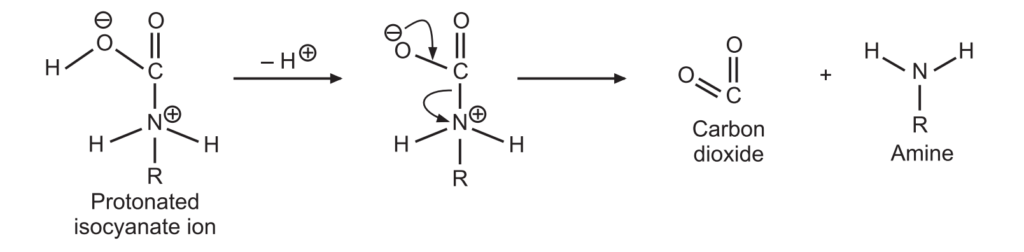
3. A protonated isocyanate ion is formed due to the addition of a water molecule. It undergoes deprotonations to give amine and carbon dioxide.
Applications of Schmidt Rearrangement
It is used to introduce amine, nitrile, amide, or imine, functional groups.
Make sure you also check our other amazing Article on : Beckmann Rearrangement