Definition: Semi-solid dosage forms are dermatological preparations intended to apply externally on the skin to produce local or systemic effects e.g. ointments, creams, gels, and pastes. They contain one or more active ingredients dissolved or uniformly dispersed in a suitable base and any suitable excipients such as emulsifiers, viscosity-increasing agents, antimicrobial agents, antioxidants, or stabilizing agents. Semisolids can adhere to the application surface for sufficiently long periods before they are washed off. This property helps prolong drug delivery at the application site. Novel semisolids are non-greasy since they are made up of water-washable bases. Hence, they cause less irritation to the skin and are superior to the conventional semisolid dosage form.
Table of Contents
IDEAL PROPERTIES OF SEMI-SOLID DOSAGE FORMS
1. Physical Properties
- They should have a smooth texture.
- They should be elegant.
- They should be non-dehydrating.
- They should be non-gritty.
- Semi-solid dosage forms possess non-greasy and non-staining properties.
- They are non-hygroscopic…
2. Physiological Properties
- They should be non-irritating.
- They should not alter skin functioning.
- They should be easily miscible with skin secretion.
- They should have a low sensitization effect.
3. Application Properties
- They should be easily applicable with efficient drug release.
- They should possess high aqueous washability.
Classification of Semi-Solid Dosage Form
Types of Semi-solid dosage form
- Ointments
- Creams
- Pastes
- Gel
- Poultices
- Plasters
Ointments: Ointments are semisolid preparations meant for external application to the skin or mucous membrane. They usually contain a medicament or medicaments dissolves, suspended, or emulsified in the base.
Creams: Creams are viscous emulsions of semisolid consistency intended for application to the skin or mucous membrane and o/w type and w/o type.
Pastes: Pastes are preparations that contain a large amount of finely powdered solids such as starch and zinc oxide. These are generally very thick and stiff.
Jellies: These are thin transparent or translucent, non-greasy preparations. They are similar to mucilages because they are prepared by using gums but they differ from mucilages in having jelly-like consistency.
Gels: These are jelly-like semisolid dispersions of the drug meant to be applied to the skin.
Suppositories: These are meant for insertion into the body cavities other than the mouth. They may be inserted into the rectum, vagina, or urethra.
Poultices: These are also known as cataplasms. They are soft viscous wet masses of solid substances.
Plasters: These are semi-solid masses applied to the skin to enable prolonged contact of the drug with the skin. or Substances intended for external application, made of such materials and consistency as to adhere to the skin and thereby attach as dressing.
Mechanism of Skin Permeation
The skin itself has two main layers, the epidermis, which is the outermost layer of the skin, covering the dermis that is the active part of the skin, holding the hair muscles, blood supply, sebaceous glands, and nerve receptors. There is a fat layer underneath the dermis. The skin is a very heterogeneous membrane and has a variety of cell types, but the layer that controls the penetration of drugs is called the stratum corneum and, despite its thickness of only 15-20 μm, it provides a very effective barrier to penetration. The permeation of the drug through the skin has several routes: transcellular, intercellular, and appendageal (through eccrine (sweat) glands or hair follicles) (Fig. 1.1).
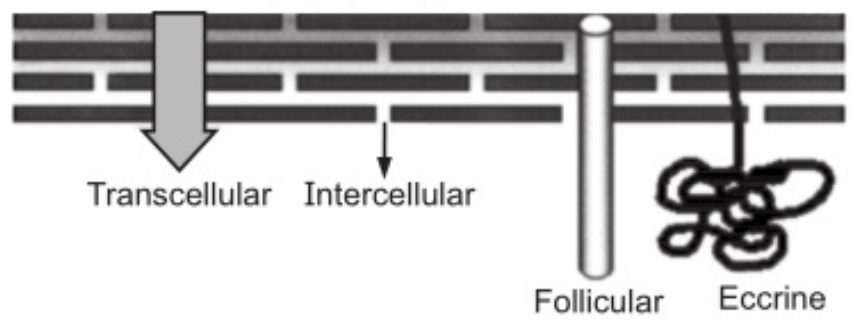
Since the appendages occupy a very low surface area, this means of permeation is less significant under normal conditions. Nevertheless, in iontophoretic delivery, this route is more significant. The intercellular spaces consist of a mixture of lipids-ceramides, free fatty acids and their esters, and cholesterol and its sulfates that are structured in bilayers. Recent developments in spectroscopic techniques give interesting insights at the molecular level that may explain the impermeability of the skin by repeated partition and diffusion across structured bilayers.
Transdermal drug permeability is influenced mainly by three factors: the mobility of the drug in the vehicle, the release of the drug from the vehicle, and drug permeation through the skin. Therefore, the researchers are challenged to come up with formulations that increase the permeability of the drug without irreversibly changing the skin barrier function. Various potential mechanisms to enhance drug penetration through the skin include directly affecting the skin and modifying the formulation so the partition, diffusion, or solubility are altered. Here we will present briefly these potential mechanisms that are interconnected with each other.
Direct Effect on the Skin
- Denaturation of intracellular keratin or modification of its conformation causes swelling and increased hydration.
- The affection of desmosomes (known as macula adherent- cell structures specialized for the cell to cell adhesin) that maintain cohesion between corneocytes (dead cells of the stratum corneum).
- Modification of lipid bilayers reduces resistance to penetration.
- Altering the solvent properties of the stratum corneum to modify drug partitioning.
- Use of solvent that can extract the lipids in the stratum corneum and decrease its resistance to penetration.
Modification of the Formulation
- Supersaturation state produced by a volatile solvent that leaves the active substance in a more thermodynamically active state.
- Choosing the enhancer molecules in the vehicle that are good solvents for the active ingredient and which enhance permeation through the skin; this way the partition of the drug into the stratum corneum will be improved.
- The diffusion of the active ingredient through the skin may be facilitated by using enhancers that create liquid pools within the bilayers like oleic acid, or disturb the bilayers uniformly as do the Azone® molecules (1-dodecyl azacyclo heptane-2-one or Lauro capram) is the first molecule specifically designed as skin permeation enhancer. Azone® serves as a surfactant and enhances the skin transport of a wide variety of drugs including steroids, antibiotics, and antiviral agents.
Method of preparation of semi-solid dosage form
1. Trituration method
2. Fusion method
3. Emulsification Method:
- Preparation of oil and aqueous phases
- Mixing of the phases
- Cooling the emulsion
- Homogenization.
4. Chemical reaction method.
Trituration Method:
It is the most commonly used for the preparation of semisolid. When the base contains soft fats and oils, or medicament is insoluble or liquid, then this method is used with a spatula or mortar and pestle.
Fusion Method:
The ingredients of the base are melted together and properly mixed to obtain a uniform product. Initially, the ingredient of a high melting point is melted. Then remaining ingredients in the base are added in the decreasing order of their melting points and melted with constant stirring. The above mixture is removed from the water bath and stirred to cool it. If the drug is soluble in the base, then its powdered form is added to the molten base. Liquid or semisolid are added at a temperature of 40°C. Insoluble additives are added in small quantities with proper stirring when the thickening of the base starts. Localized cooling of the molten base and vigorous stirring should be avoided to prevent aeration of the ointment.
Emulsification Method
Preparation of Oil and Aqueous Phases: Place the ingredients of the oil phase into the stainless steel steam-jacketed kettle and melt them whilst mixing. Filter the oil phase through several layers of cheesecloth to remove any foreign matter. Heat the emulsion mixing kettle to the temperature of the oil phase. This avoids congealing of the higher melting component. Transfer the oil phase into the emulsion mixing kettle. Dissolve the ingredient of the aqueous phase in purified water and filter the solution. A soluble drug that is thermostable may be added to the aqueous phase in this step.
The phases are usually mixed at a temperature of 70 to 72°C because at this temperature intimate mixing of the liquid phases can occur. The properties of some emulsions depend on the temperature at which the phases are mixed.
Three ways of mixing the phases:
- The simultaneous blending of the phases.
- Addition of the discontinuous phase to the continuous phase.
- Addition of the continuous phase to the discontinuous phase.
Types of equipment used for mixing of phases:
Agitator mixers: Sigma mixer and planetary mixer.
Shear mixers: Triple roller mill and Colloidal mill.
Chemical reaction:
This method is used to prepare several types of ointments. This method involves both fusion and mechanical mixing. The best example of this method is Iodine ointment.
Chemical Reaction Method Procedure for iodine ointment: Powder iodine in a mortar and pestle and add it to Arachis oil taken in a flask. Heat the mixture to 50°C with occasional stirring until greenish-black color appears. Add yellow soft paraffin to the above mixture and heat it to 40°C with mixing. Cool the Ointment.
Make sure you also check our other amazing Article on : Suspensions
